

ISSN 2954-467X

Saldívar Cerón Héctor Iván
1 Carrera de Médico Cirujano, Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México, Tlalnepantla 54090, México.
2 Red de Medicina para la Educación y Desarrollo y la Investigación Científica de Iztacala (Red MEDICI), Carrera Médico Cirujano, Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México, Tlalnepantla 54090, México.
3 Laboratorio 14, Unidad de Biomedicina, Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México, Tlalnepantla 54090, México.
Contact: Saldívar Cerón Héctor Iván. mail: ivansaldi@iztacala.unam.mx. Avenida de los Barrios 112, 54090 Tlalnepantla de Baz, Mexico. Phone: +52 55 79801550
Article History: Received September 21, 2022. Revised September 26, 2022. Accepted September 30, 2022. Available online October 1, 2022.
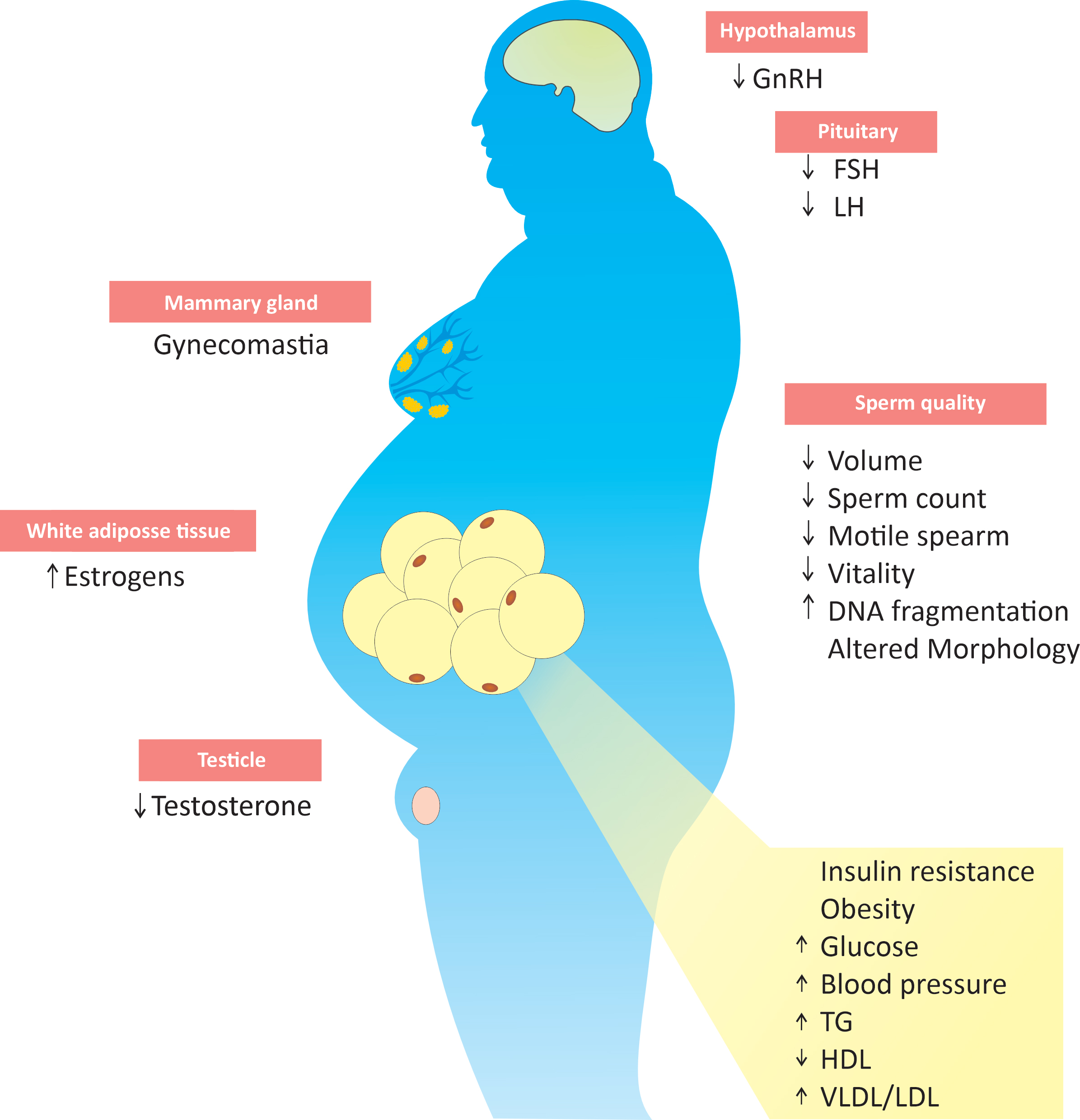
Figure 1. The impact of the metabolic syndrome on male fertility.
[1] Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int J Sports Med. 2021 Mar;42(3):199-214. doi: 10.1055/a-1263-0898. Epub 2020 Oct 19. PMID: 33075830.
[2] Belete, R., Ataro, Z., Abdu, A. et al. Global prevalence of metabolic syndrome among patients with type I diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr 13, 25 (2021). doi.org/10.1186/s13098-021-00641-8.
[3] Gutiérrez-Solis AL, Datta Banik S, Méndez-González RM. Prevalence of Metabolic Syndrome in Mexico: A Systematic Review and Meta-Analysis. Metab Syndr Relat Disord. 2018 Oct;16(8):395-405. doi: 10.1089/met.2017.0157. Epub 2018 Jul 31. PMID: 30063173.
[4] Leisegang K, Sengupta P, Agarwal A, Henkel R. Obesity and male infertility: Mechanisms and management. Andrologia. 2021 Feb;53(1):e13617. doi: 10.1111/and.13617. Epub 2020 May 12. PMID: 32399992).
[5] Salvio G, Ciarloni A, Cutini M, Delli Muti N, Finocchi F, Perrone M, Rossi S, Balercia G. Metabolic Syndrome and Male Fertility: Beyond Heart Consequences of a Complex Cardiometabolic Endocrinopathy. Int J Mol Sci. 2022 May 14;23(10):5497. doi: 10.3390/ijms23105497. PMID: 35628307; PMCID: PMC9143238.
[6] Martins AD, Majzoub A, Agawal A. Metabolic Syndrome and Male Fertility. World J Mens Health. 2019 May;37(2):113-127. doi: 10.5534/wjmh.180055. Epub 2018 Oct 22. PMID: 30350486; PMCID: PMC6479081.
[7] Le MT, Tran NQT, Nguyen ND, Nguyen QHV. The Prevalence and Components of Metabolic Syndrome in Men from Infertile Couples and Its Relation on Semen Analysis. Diabetes Metab Syndr Obes. 2021 Mar 29;14:1453-1463. doi: 10.2147/DMSO.S302575. PMID: 33824599; PMCID: PMC8018567.
[8] Zhao L, Pang A. Effects of Metabolic Syndrome on Semen Quality and Circulating Sex Hormones: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2020 Aug 11;11:428. doi: 10.3389/fendo.2020.00428. PMID: 32849258; PMCID: PMC7431460.
[9] Andersen E, Juhl CR, Kjøller ET, Lundgren JR, Janus C, Dehestani Y, Saupstad M, Ingerslev LR, Duun OM, Jensen SBK, Holst JJ, Stallknecht BM, Madsbad S, Torekov SS, Barrès R. Sperm count is increased by diet-induced weight loss and maintained by exercise or GLP-1 analogue treatment: a randomized controlled trial. Hum Reprod. 2022 Jun 30;37(7):1414-1422. doi: 10.1093/humrep/deac096. PMID: 35580859; PMCID: PMC9247415.
[10] Sultan S, Patel AG, El-Hassani S, Whitelaw B, Leca BM, Vincent RP, le Roux CW, Rubino F, Aywlin SJB, Dimitriadis GK. Male Obesity Associated Gonadal Dysfunction and the Role of Bariatric Surgery. Front Endocrinol (Lausanne). 2020 Jun 19;11:408. doi: 10.3389/fendo.2020.00408. PMID: 32636807; PMCID: PMC7318874.
[11] Dupont J, Pollet-Villard X, Reverchon M, Mellouk N, Levy R. Adipokines in human reproduction. Horm Mol Biol Clin Investig. 2015 Oct;24(1):11-24. doi: 10.1515/hmbci-2015-0034. PMID: 26574894.
[12] Liu Y, Ding Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction. 2017 Oct;154(4):R123-R131. doi: 10.1530/REP-17-0161. Epub 2017 Jul 26. PMID: 28747541.
[13] Carrageta DF, Oliveira PF, Alves MG, Monteiro MP. Obesity and male hypogonadism: Tales of a vicious cycle. Obes Rev. 2019 Aug;20(8):1148-1158. doi: 10.1111/obr.12863. Epub 2019 Apr 29. PMID: 31035310.
[14] Kahn BE, Brannigan RE. Obesity and male infertility. Curr Opin Urol. 2017 Sep;27(5):441-445. doi: 10.1097/MOU.0000000000000417. PMID: 28661897.
[15] Elmas MA, Ozakpinar OB, Kolgazi M, Sener G, Arbak S, Ercan F. Exercise improves testicular morphology and oxidative stress parameters in rats with testicular damage induced by a high-fat diet. Andrologia. 2022 Sep 22:e14600. doi: 10.1111/and.14600. Epub ahead of print. PMID: 36146902.
[16] Ribeiro JC, Martins AD, Jarak I, Carvalho RA, Alves MG, Oliveira PF. Exenatide and Dapagliflozin Combination Enhances Sertoli Cell Secretion of Key Metabolites for Spermatogenesis. Biomedicines. 2022 May 11;10(5):1115. doi: 10.3390/biomedicines10051115. PMID: 35625851; PMCID: PMC9139030.
[17] Salas-Huetos A, Maghsoumi-Norouzabad L, James ER, Carrell DT, Aston KI, Jenkins TG, Becerra-Tomás N, Javid AZ, Abed R, Torres PJ, Luque EM, Ramírez ND, Martini AC, Salas-Salvadó J. Male adiposity, sperm parameters and reproductive hormones: An updated systematic review and collaborative meta-analysis. Obes Rev. 2021 Jan;22(1):e13082. doi: 10.1111/obr.13082. Epub 2020 Jul 23. PMID: 32705766.
[18] Tchernof A, Després JP. Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm Metab Res. 2000 Nov-Dec;32(11-12):526-36. doi: 10.1055/s-2007-978681. PMID: 11246820.
[19] Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013 Jan;93(1):359-404. doi: 10.1152/physrev.00033.2011. PMID: 23303913.
[20] Barbagallo F, Condorelli RA, Mongioì LM, Cannarella R, Cimino L, Magagnini MC, Crafa A, La Vignera S, Calogero AE. Molecular Mechanisms Underlying the Relationship between Obesity and Male Infertility. Metabolites. 2021 Dec 4;11(12):840. doi: 10.3390/metabo11120840. PMID: 34940598; PMCID: PMC8706114.
[21] Yuxin L, Chen L, Xiaoxia L, Yue L, Junjie L, Youzhu L, Huiliang Z, Qicai L. Research Progress on the Relationship between Obesity-Inflammation-Aromatase Axis and Male Infertility. Oxid Med Cell Longev. 2021 Feb 8;2021:6612796. doi: 10.1155/2021/6612796. PMID: 33628365; PMCID: PMC7884171.
[22] Cooke PS, Heine PA, Taylor JA, Lubahn DB. The role of estrogen and estrogen receptor-alpha in male adipose tissue. Mol Cell Endocrinol. 2001 Jun 10;178(1-2):147-54. doi: 10.1016/s0303-7207(01)00414-2. PMID: 11403904.
[23] Kanakis GA, Nordkap L, Bang AK, Calogero AE, Bártfai G, Corona G, Forti G, Toppari J, Goulis DG, Jørgensen N. EAA clinical practice guidelines-gynecomastia evaluation and management. Andrology. 2019 Nov;7(6):778-793. doi: 10.1111/andr.12636. Epub 2019 May 16. PMID: 31099174.
[24] Vandeven HA, Pensler JM. Gynecomastia. 2022 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 28613563.
[25] Zsido RG, Heinrich M, Slavich GM, Beyer F, Kharabian Masouleh S, Kratzsch J, Raschpichler M, Mueller K, Scharrer U, Löffler M, Schroeter ML, Stumvoll M, Villringer A, Witte AV, Sacher J. Association of Estradiol and Visceral Fat With Structural Brain Networks and Memory Performance in Adults. JAMA Netw Open. 2019 Jun 5;2(6):e196126. doi: 10.1001/jamanetworkopen.2019.6126. PMID: 31225892; PMCID: PMC6593958.
[26] Carrageta DF, Oliveira PF, Alves MG, Monteiro MP. Obesity and male hypogonadism: Tales of a vicious cycle. Obes Rev. 2019 Aug;20(8):1148-1158. doi: 10.1111/obr.12863. Epub 2019 Apr 29. PMID: 31035310.
[27] Yang C, Li P, Li Z. Clinical application of aromatase inhibitors to treat male infertility. Hum Reprod Update. 2021 Dec 21;28(1):30-50. doi: 10.1093/humupd/dmab036. PMID: 34871401.
[28] Lo EM, Rodriguez KM, Pastuszak AW, Khera M. Alternatives to Testosterone Therapy: A Review. Sex Med Rev. 2018 Jan;6(1):106-113. doi: 10.1016/j.sxmr.2017.09.004. Epub 2017 Nov 27. PMID: 29174957.
[29] Aydogdu A, Swerdloff RS. Emerging medication for the treatment of male hypogonadism. Expert Opin Emerg Drugs. 2016 Sep;21(3):255-66. doi: 10.1080/14728214.2016.1226799. PMID: 27552127.
[30] Ring JD, Lwin AA, Köhler TS. Current medical management of endocrine-related male infertility. Asian J Androl. 2016 May-Jun;18(3):357-63. doi: 10.4103/1008-682X.179252. PMID: 27098657; PMCID: PMC4854080.
[31] Rowland DL, McNabney SM, Mann AR. Sexual Function, Obesity, and Weight Loss in Men and Women. Sex Med Rev. 2017 Jul;5(3):323-338. doi: 10.1016/j.sxmr.2017.03.006. Epub 2017 Apr 26. PMID: 28456610.
[32] Abrahamian H, Kautzky-Willer A. Sexualität bei Übergewicht und Adipositas [Sexuality in overweight and obesity]. Wien Med Wochenschr. 2016 Mar;166(3-4):121-8. German. doi: 10.1007/s10354-016-0430-9. Epub 2016 Jan 26. PMID: 26811242.
[33] Bates JN, Pastuszak AW, Khera M. Effect of Body Weight on Sexual Function in Men and Women. Curr Sex Health Rep. 2019 Mar;11(1):52-59. Epub 2019 Jan 19. PMID: 31576197; PMCID: PMC6771291.
[34] Conason A, McClure Brenchley KJ, Pratt A, Geliebter A. Sexual life after weight loss surgery. Surg Obes Relat Dis. 2017 May;13(5):855-861. doi: 10.1016/j.soard.2017.01.014. Epub 2017 Jan 9. PMID: 28366669.
[35] Kałużna M, Nomejko A, Słowińska A, Wachowiak-Ochmańska K, Pikosz K, Ziemnicka K, Ruchała M. Lower sexual satisfaction in women with polycystic ovary syndrome and metabolic syndrome. Endocr Connect. 2021 Aug 30;10(9):1035-1044. doi: 10.1530/EC-21-0257. PMID: 34319905; PMCID: PMC8428045.