

ISSN 2954-467X

Saldívar Cerón Héctor Iván
Saldívar Cerón Héctor Iván1,3, 0000-0002-9125-9100; Castañeda Ramírez Ari Evelyn1, 0000-0002-1465-8255; Quiñones Lara Efrén1, 0000-0001-5577-0908; Vargas Camacho Jorge Arturo1, 0000-0002-7727-1576; López Desidero Nely Gisela1,2,4, 0000-0002-5107-6158.
1 Carrera de Médico Cirujano, Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México, Tlalnepantla 54090, México.
2 Red de Medicina para la Educación y Desarrollo y la Investigación Científica de Iztacala (Red MEDICI), Carrera Médico Cirujano, Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México, Tlalnepantla 54090, México.
3 Laboratorio 14, Unidad de Biomedicina (UBIMED), Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México, Tlalnepantla 54090, México.
4 Laboratorio de Medicina de Conservación, Escuela Superior de Medicina, Instituto Politécnico Nacional, Ciudad de México, 11340, México.
NOTE: The numbers following the affiliation markers are the author's ORCID iD.
Contact: Saldívar Cerón Héctor Iván. mail: ivansaldi@iztacala.unam.mx. Avenida de los Barrios 112, 54090 Tlalnepantla de Baz, México. Phone: +52 55 7980 1550.
Article History: Received February 8, 2023. Revised February 15, 2023. Accepted February 22, 2023. Available online April 26, 2023.
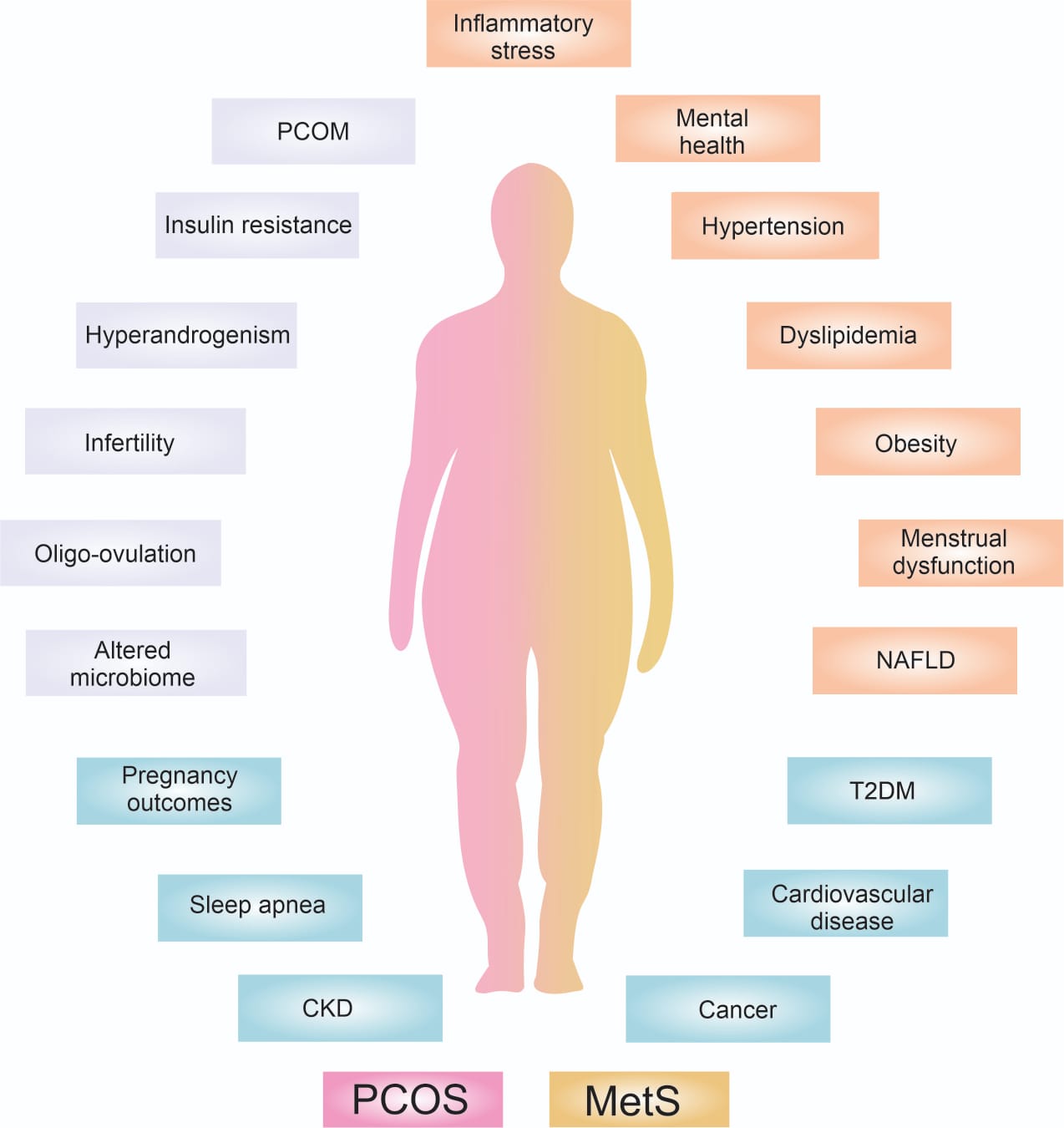
Figure 1. Interrelationship between PCOS and MetS. The interrelation between PCOS and MetS is a result of a vicious circle where excessive visceral adipose tissue increases androgen production, which in turn can trigger PCOS. However, women with PCOS also have a higher risk of developing obesity due to insulin resistance. As a result, MetS and PCOS feed each other. This potentiates the presence of short-term comorbidities such as mental health problems, menstrual disorders, infertility, acanthosis nigricans, etc., and in the long term: type 2 diabetes (T2DM), chronic kidney disease (CKD), increased risk of cancer, cardiovascular diseases, obstructive sleep apnea, non-alcoholic fatty liver disease and liver cirrhosis, leading to patient death. It is important to identify and refer both syndromes to address these comorbidities and prevent any long-term health problems.
[1] Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009 Feb;91(2):456-88. doi: 10.1016/j.fertnstert.2008.06.035. Epub 2008 Oct 23. PMID: 18950759
[2] Yilmaz B, Vellanki P, Ata B, Yildiz BO. Metabolic syndrome, hypertension, and hyperlipidemia in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 2018 Feb;109(2):356-364.e32. doi: 10.1016/j.fertnstert.2017.10.018. Epub 2018 Jan 11. PMID: 29331234; PMCID: PMC5983376.
[3] Sam S, Dunaif A. Polycystic ovary syndrome: syndrome XX? Trends Endocrinol Metab. 2003 Oct;14(8):365-70. doi: 10.1016/j.tem.2003.08.002. PMID: 14516934.
[4] Zehravi M, Maqbool M, Ara I. Polycystic ovary syndrome and infertility: an update. Int J Adolesc Med Health. 2021 Jul 22;34(2):1-9. doi: 10.1515/ijamh-2021-0073. PMID: 34293835.
[5] The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum. Reprod. 19, 41–47 (2004).
[6] Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006 Nov;91(11):4237-45. doi: 10.1210/jc.2006-0178. Epub 2006 Aug 29. PMID: 16940456.
[7] Steering Committee of the National Institutes of Health Evidence-Based Methodology Workshop on Polycystic Ovary Syndrome. Evidence-based Methodology Workshop on Polycystic Ovary Syndrome. Final Report. https://prevention.nih.gov/docs/programs/pcos/FinalReport.pdf (National Institute of Health, Bethesda, MD, USA, 2012).
[8] Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018 May;14(5):270-284. doi: 10.1038/nrendo.2018.24. Epub 2018 Mar 23. PMID: 29569621.
[9] Marchesan LB, Ramos RB, Spritzer PM. Metabolic Features of Women With Polycystic Ovary Syndrome in Latin America: A Systematic Review. Front Endocrinol (Lausanne). 2021 Oct 19;12:759835. doi: 10.3389/fendo.2021.759835. PMID: 34737723; PMCID: PMC8562723.
[10] Layacha SY, Biswas DA. Women With Polycystic Ovary Syndrome: A Review of Susceptibility to Type 2 Diabetes. Cureus. 2023 Jan 5;15(1):e33390. doi: 10.7759/cureus.33390. PMID: 36751233; PMCID: PMC9897680.
[11] Yu J, Zhou Y, Ding J, Zhang D, Yu C, Huang H. Characteristics and possible mechanisms of metabolic disorder in overweight women with polycystic ovary syndrome. Front Endocrinol (Lausanne). 2023 Jan 12;13:970733. doi: 10.3389/fendo.2022.970733. PMID: 36714563; PMCID: PMC9878688.
[12] Azziz R. PCOS in 2015: New insights into the genetics of polycystic ovary syndrome. Nat Rev Endocrinol. 2016 Feb;12(2):74-5. doi: 10.1038/nrendo.2015.230. Epub 2016 Jan 4. Erratum in: Nat Rev Endocrinol. 2016 Mar;12(3):183. PMID: 26729036.
[13] Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA, Okeya BL, Abbott DH, Chazenbalk GD. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J Clin Endocrinol Metab. 2016 Nov;101(11):4178-4188. doi: 10.1210/jc.2016-2586. Epub 2016 Aug 29. PMID: 27571186; PMCID: PMC5095243.
[14] Panidis D, Tziomalos K, Misichronis G, Papadakis E, Betsas G, Katsikis I, Macut D. Insulin resistance and endocrine characteristics of the different phenotypes of polycystic ovary syndrome: a prospective study. Hum Reprod. 2012 Feb;27(2):541-9. doi: 10.1093/humrep/der418. Epub 2011 Dec 5. PMID: 22144419.
[15] Escobar-Morreale HF, Samino S, Insenser M, Vinaixa M, Luque-Ramírez M, Lasunción MA, Correig X. Metabolic heterogeneity in polycystic ovary syndrome is determined by obesity: plasma metabolomic approach using GC-MS. Clin Chem. 2012 Jun;58(6):999-1009. doi: 10.1373/clinchem.2011.176396. Epub 2012 Mar 16. PMID: 22427353.
[16] Welt CK, Gudmundsson JA, Arason G, Adams J, Palsdottir H, Gudlaugsdottir G, Ingadottir G, Crowley WF. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: the impact of weight on phenotype and metabolic features. J Clin Endocrinol Metab. 2006 Dec;91(12):4842-8. doi: 10.1210/jc.2006-1327. Epub 2006 Sep 26. PMID: 17003085.
[17] Mirza FG, Tahlak MA, Rjeili RB, Hazari K, Ennab F, Hodgman C, Khamis AH, Atiomo W. Polycystic Ovarian Syndrome (PCOS): Does the Challenge End at Conception? Int J Environ Res Public Health. 2022 Nov 12;19(22):14914. doi: 10.3390/ijerph192214914. PMID: 36429632; PMCID: PMC9690374.
[18] Chakraborty P, Goswami SK, Rajani S, Sharma S, Kabir SN, Chakravarty B, Jana K. Recurrent pregnancy loss in polycystic ovary syndrome: role of hyperhomocysteinemia and insulin resistance. PLoS One. 2013 May 21;8(5):e64446. doi: 10.1371/journal.pone.0064446. PMID: 23700477; PMCID: PMC3660299.
[19] Zhai J, Li Z, Zhou Y, Yang X. The role of plasminogen activator inhibitor-1 in gynecological and obstetrical diseases: An update review. J Reprod Immunol. 2022 Mar;150:103490. doi: 10.1016/j.jri.2022.103490. Epub 2022 Jan 29. PMID: 35121287.
[20] Fernando M, Ellery SJ, Marquina C, Lim S, Naderpoor N, Mousa A. Vitamin D-Binding Protein in Pregnancy and Reproductive Health. Nutrients. 2020 May 20;12(5):1489. doi: 10.3390/nu12051489. PMID: 32443760; PMCID: PMC7285222.
[21] Clifford K, Rai R, Watson H, Franks S, Regan L. Does suppressing luteinising hormone secretion reduce the miscarriage rate? Results of a randomised controlled trial. BMJ. 1996 Jun 15;312(7045):1508-11. doi: 10.1136/bmj.312.7045.1508. PMID: 8646142; PMCID: PMC2351255.
[22] Heijnen EM, Eijkemans MJ, Hughes EG, Laven JS, Macklon NS, Fauser BC. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006 Jan-Feb;12(1):13-21. doi: 10.1093/humupd/dmi036. Epub 2005 Aug 25. PMID: 16123051.
[23] Bond R, Pace R, Rahme E, Dasgupta K. Diabetes risk in women with gestational diabetes mellitus and a history of polycystic ovary syndrome: a retrospective cohort study. Diabet Med. 2017 Dec;34(12):1684-1695. doi: 10.1111/dme.13444. Epub 2017 Sep 1. PMID: 28782842.
[24] Toulis KA, Goulis DG, Kolibianakis EM, Venetis CA, Tarlatzis BC, Papadimas I. Risk of gestational diabetes mellitus in women with polycystic ovary syndrome: a systematic review and a meta-analysis. Fertil Steril. 2009 Aug;92(2):667-77. doi: 10.1016/j.fertnstert.2008.06.045. Epub 2008 Aug 16. PMID: 18710713.
[25] Turhan NO, Seçkin NC, Aybar F, Inegöl I. Assessment of glucose tolerance and pregnancy outcome of polycystic ovary patients. Int J Gynaecol Obstet. 2003 May;81(2):163-8. doi: 10.1016/s0020-7292(03)00003-1. PMID: 12706273.
[26] Wortsman J, de Angeles S, Futterweit W, Singh KB, Kaufmann RC. Gestational diabetes and neonatal macrosomia in the polycystic ovary syndrome. J Reprod Med. 1991 Sep;36(9):659-61. PMID: 1774730.
[27] Pan H, Xian P, Yang D, Zhang C, Tang H, He X, Lin H, Wen X, Ma H, Lai M. Polycystic ovary syndrome is an independent risk factor for hypertensive disorders of pregnancy: A systematic review, meta-analysis, and meta-regression. Endocrine. 2021 Dec;74(3):518-529. doi: 10.1007/s12020-021-02886-9. Epub 2021 Oct 16. PMID: 34655376.
[28] Hodgman C, Khan GH, Atiomo W. Coenzyme A Restriction as a Factor Underlying Pre-Eclampsia with Polycystic Ovary Syndrome as a Risk Factor. Int J Mol Sci. 2022 Mar 3;23(5):2785. doi: 10.3390/ijms23052785. PMID: 35269927; PMCID: PMC8911031.
[29] de Vries MJ, Dekker GA, Schoemaker J. Higher risk of preeclampsia in the polycystic ovary syndrome. A case control study. Eur J Obstet Gynecol Reprod Biol. 1998 Jan;76(1):91-5. doi: 10.1016/s0301-2115(97)00164-4. PMID: 9481555.
[30] Maru L, Verma M, Jinsiwale N. Homocysteine as Predictive Marker for Pregnancy-Induced Hypertension-A Comparative Study of Homocysteine Levels in Normal Versus Patients of PIH and Its Complications. J Obstet Gynaecol India. 2016 Oct;66(Suppl 1):167-71. doi: 10.1007/s13224-015-0832-4. Epub 2016 Feb 26. PMID: 27651597; PMCID: PMC5016440.
[31] Yamamoto M, Feigenbaum SL, Crites Y, Escobar GJ, Yang J, Ferrara A, Lo JC. Risk of preterm delivery in non-diabetic women with polycystic ovarian syndrome. J Perinatol. 2012 Oct;32(10):770-6. doi: 10.1038/jp.2011.194. Epub 2012 Jan 19. PMID: 22261835; PMCID: PMC3570271.
[32] Altieri P, Gambineri A, Prontera O, Cionci G, Franchina M, Pasquali R. Maternal polycystic ovary syndrome may be associated with adverse pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol. 2010 Mar;149(1):31-6. doi: 10.1016/j.ejogrb.2009.11.010. Epub 2010 Jan 6. PMID: 20056308.
[33] De Frène V, Vansteelandt S, T'Sjoen G, Gerris J, Somers S, Vercruysse L, De Sutter P. A retrospective study of the pregnancy, delivery and neonatal outcome in overweight versus normal weight women with polycystic ovary syndrome. Hum Reprod. 2014 Oct 10;29(10):2333-8. doi: 10.1093/humrep/deu154. Epub 2014 Jun 24. PMID: 24963163.
[34] Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA)--a novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses. 2012 Jul;79(1):104-12. doi: 10.1016/j.mehy.2012.04.016. Epub 2012 Apr 27. PMID: 22543078.
[35] Mukherjee AG, Wanjari UR, Kannampuzha S, Murali R, Namachivayam A, Ganesan R, Dey A, Babu A, Renu K, Vellingiri B, Ramanathan G, Priya Doss C G, Elsherbiny N, Elsherbini AM, Alsamman AM, Zayed H, Gopalakrishnan AV. The Implication of Mechanistic Approaches and the Role of the Microbiome in Polycystic Ovary Syndrome (PCOS): A Review. Metabolites. 2023 Jan 14;13(1):129. doi: 10.3390/metabo13010129. PMID: 36677054; PMCID: PMC9863528.
[36] Lüll K, Arffman RK, Sola-Leyva A, Molina NM, Aasmets O, Herzig KH, Plaza-Díaz J, Franks S, Morin-Papunen L, Tapanainen JS, Salumets A, Altmäe S, Piltonen TT, Org E. The Gut Microbiome in Polycystic Ovary Syndrome and Its Association with Metabolic Traits. J Clin Endocrinol Metab. 2021 Mar 8;106(3):858-871. doi: 10.1210/clinem/dgaa848. Erratum in: J Clin Endocrinol Metab. 2022 May 17;107(6):e2660. PMID: 33205157.
[37] Suturina L, Belkova N, Igumnov I, Lazareva L, Danusevich I, Nadeliaeva I, Sholokhov L, Rashidova M, Belenkaya L, Belskikh A, Sharifulin E, Ievleva K, Babaeva N, Egorova I, Salimova M, Kuzmin M, Tiumentseva D, Klimenko E, Sidorova T, Atalyan A. Polycystic Ovary Syndrome and Gut Microbiota: Phenotype Matters. Life (Basel). 2022 Dec 20;13(1):7. doi: 10.3390/life13010007. PMID: 36675956; PMCID: PMC9861125.
[38] Liu, Q., Xie, Y., Qu, L., Zhang, M., & Mo, Z.(2019). Dyslipidemia involvement in the development of polycystic ovary syndrome. Taiwanese Journal of Obstetrics and Gynecology, 58(4), 447-453. doi: 10.1016/ j.tjog.2019.05.00320:14.
[39] de Medeiros SF, Yamamoto MMW, de Medeiros MAS, Yamamoto AKLW, Barbosa BB. Polycystic ovary syndrome and risks for COVID-19 infection: A comprehensive review : PCOS and COVID-19 relationship. Rev Endocr Metab Disord. 2022 Apr;23(2):251-264. doi: 10.1007/s11154-022-09715-y. Epub 2022 Feb 26. PMID: 35218458; PMCID: PMC8881900.
[40] Bajgain KT, Badal S, Bajgain BB, Santana MJ. Prevalence of comorbidities among individuals with COVID-19: A rapid review of current literature. Am J Infect Control. 2021 Feb;49(2):238-246. doi: 10.1016/j.ajic.2020.06.213. Epub 2020 Jul 10. PMID: 32659414; PMCID: PMC7351042.
[41] Klonoff DC, Umpierrez GE. Letter to the Editor: COVID-19 in patients with diabetes: Risk factors that increase morbidity. Metabolism. 2020 Jul;108:154224. doi: 10.1016/j.metabol.2020.154224. Epub 2020 Apr 7. PMID: 32275971; PMCID: PMC7138381.
[42] Glendining KA, Campbell RE. Recent advances in emerging PCOS therapies. Curr Opin Pharmacol. 2023 Jan 6;68:102345. doi: 10.1016/j.coph.2022.102345. Epub ahead of print. PMID: 36621270.
[43] Ghasemi Tehrani H, Aasasi K, Mardanian F, Mehrabian F, Movahedi M, Naghshineh E. Evaluation of The Effect of Letrozole in the Ovarian Hyperstimulation Syndrome Prevention in Participants at Risk of Treatment with Ovulation-Stimulating Drugs:A Randomized Controlled Trial. Rep Biochem Mol Biol. 2022 Oct;11(3):386-393. doi: 10.52547/rbmb.11.3.386. PMID: 36718297; PMCID: PMC9883038.
[44] Gariani K, Hugon-Rodin J, Philippe J, Righini M, Blondon M. Association between polycystic ovary syndrome and venous thromboembolism: A systematic review and meta-analysis. Thromb Res. 2020 Jan;185:102-108. doi: 10.1016/j.thromres.2019.11.019. Epub 2019 Nov 20. PMID: 31790999.
[45] Carvalho MJ, Subtil S, Rodrigues Â, Oliveira J, Figueiredo-Dias M. Controversial association between polycystic ovary syndrome and breast cancer. Eur J Obstet Gynecol Reprod Biol. 2019 Dec;243:125-132. doi: 10.1016/j.ejogrb.2019.10.011. Epub 2019 Oct 15. PMID: 31693949.
[46] Elenis E, Desroziers E, Persson S, Sundström Poromaa I, Campbell RE. Early initiation of anti-androgen treatment is associated with increased probability of spontaneous conception leading to childbirth in women with polycystic ovary syndrome: a population-based multiregistry cohort study in Sweden. Hum Reprod. 2021 Apr 20;36(5):1427-1435. doi: 10.1093/humrep/deaa357. PMID: 33454768; PMCID: PMC8058592.
[47] Bertoldo MJ, Caldwell ASL, Riepsamen AH, Lin D, Gonzalez MB, Robker RL, Ledger WL, Gilchrist RB, Handelsman DJ, Walters KA. A Hyperandrogenic Environment Causes Intrinsic Defects That Are Detrimental to Follicular Dynamics in a PCOS Mouse Model. Endocrinology. 2019 Mar 1;160(3):699-715. doi: 10.1210/en.2018-00966. PMID: 30657917.
[48] Moretti C, Guccione L, Di Giacinto P, Simonelli I, Exacoustos C, Toscano V, Motta C, De Leo V, Petraglia F, Lenzi A. Combined Oral Contraception and Bicalutamide in Polycystic Ovary Syndrome and Severe Hirsutism: A Double-Blind Randomized Controlled Trial. J Clin Endocrinol Metab. 2018 Mar 1;103(3):824-838. doi: 10.1210/jc.2017-01186. PMID: 29211888.
[49] George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, Veldhuis J, Skorupskaite K, Anderson RA, McIntosh S, Webber L. Neurokinin B Receptor Antagonism in Women With Polycystic Ovary Syndrome: A Randomized, Placebo-Controlled Trial. J Clin Endocrinol Metab. 2016 Nov;101(11):4313-4321. doi: 10.1210/jc.2016-1202. Epub 2016 Jul 26. PMID: 27459523.
[50] Abbara A, Eng PC, Phylactou M, Clarke SA, Richardson R, Sykes CM, Phumsatitpong C, Mills E, Modi M, Izzi-Engbeaya C, Papadopoulou D, Purugganan K, Jayasena CN, Webber L, Salim R, Owen B, Bech P, Comninos AN, McArdle CA, Voliotis M, Tsaneva-Atanasova K, Moenter S, Hanyaloglu A, Dhillo WS. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J Clin Invest. 2020 Dec 1;130(12):6739-6753. doi: 10.1172/JCI139681. PMID: 33196464; PMCID: PMC7685751.
[51] Fraser GL, Obermayer-Pietsch B, Laven J, Griesinger G, Pintiaux A, Timmerman D, Fauser BCJM, Lademacher C, Combalbert J, Hoveyda HR, Ramael S. Randomized Controlled Trial of Neurokinin 3 Receptor Antagonist Fezolinetant for Treatment of Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2021 Aug 18;106(9):e3519-e3532. doi: 10.1210/clinem/dgab320. PMID: 34000049; PMCID: PMC8372662.
[52] Wang Y, Zeng Z, Zhao S, Tang L, Yan J, Li N, Zou L, Fan X, Xu C, Huang J, Xia W, Zhu C, Rao M. Humanin Alleviates Insulin Resistance in Polycystic Ovary Syndrome: A Human and Rat Model-Based Study. Endocrinology. 2021 Aug 1;162(8):bqab056. doi: 10.1210/endocr/bqab056. PMID: 33693742.
[53] Han Y, Li Y, He B. GLP-1 receptor agonists versus metformin in PCOS: a systematic review and meta-analysis. Reprod Biomed Online. 2019 Aug;39(2):332-342. doi: 10.1016/j.rbmo.2019.04.017. Epub 2019 Apr 25. PMID: 31229399.
[54] Sinha B, Ghosal S. A Meta-Analysis of the Effect of Sodium Glucose Cotransporter-2 Inhibitors on Metabolic Parameters in Patients With Polycystic Ovary Syndrome. Front Endocrinol (Lausanne). 2022 Feb 21;13:830401. doi: 10.3389/fendo.2022.830401. PMID: 35265039; PMCID: PMC8900375.