

ISSN 2954-467X

Ronny Kershenovich Sefchovich
Ronny Kershenovich Sefchovich1, 0009-0000-2539-3013; Héctor Oviedo Cruz2, 0000-0001-8781-5592; Marcela Fragoso Benitez3, 0000-0002-4218-9951; Leonardo Pérez Mejía4, 0009-0001-5797-8857; Rolando Álvarez Valero1, 0009-0002-4790-1634.
1 American British Cowdray Medical Center (Centro Médico ABC).
2 Centro Médico Para Atención Fetal Especializada (CEMAFE).
3 GD Technologies.
4 Genos Médica.
NOTE: The numbers following the affiliation markers are the author's ORCID iD.
Contact: Dr. Ronny Kershenovich Sefcho. mail: genetista@me.com. American British Cowdray Medical Center, Av. Carlos Graef Fernández, 154, Colonia Santa Fe, Cuajimalpa, CP 05300, Mexico City, CEGOP Building, Lobby, Office 1B. Phone: +52-5516647227.
Article History: Received July 11th, 2023. Revised July 21th, 2023. Accepted July 25th, 2023. Available online August 29th, 2023.
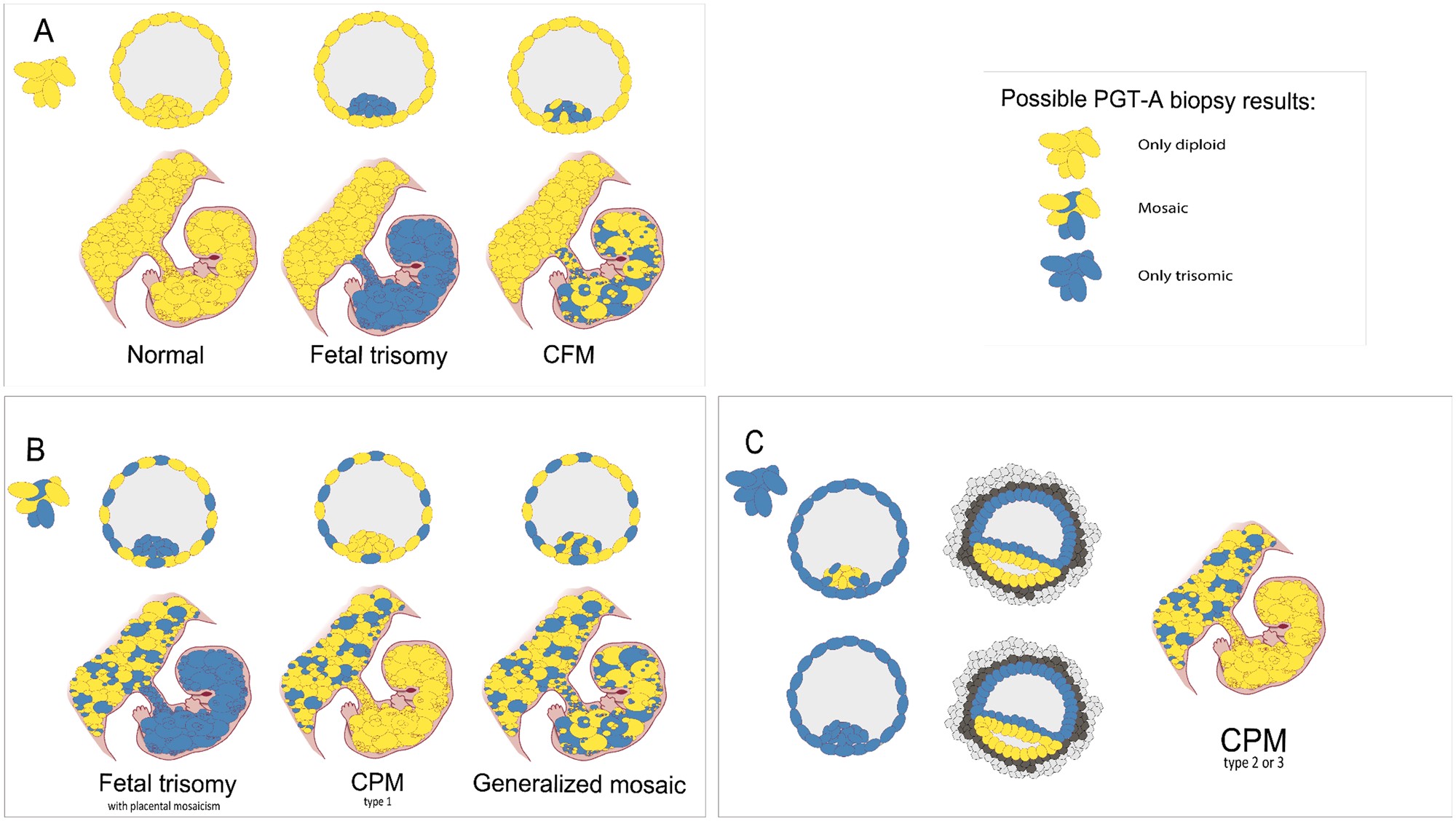
Figure 1. From blastocyst cells to prenatal scenario. (A) Three different scenarios arise if only diploid cells are retrieved through biopsy in blastocyst stage. (B) When both diploid and trisomic cells are biopsied, three different scenarios can also arise. If the mosaicism is only found in the trophectoderm and not within the inner cell mass (ICM), confined placental mosaicim (CPM) type 1 develops. (C) If all biopsied cells appear to be trisomic, even in the ICM, as a result of trisomic rescue, the epiblast will eliminate the trisomic cells and will only consist of diploid cells, thus CPM type 2 or 3 can develop.Taken from Toutain et al., 2018.
Figure use license: https://doi.org/10.1093/humupd/dmab009
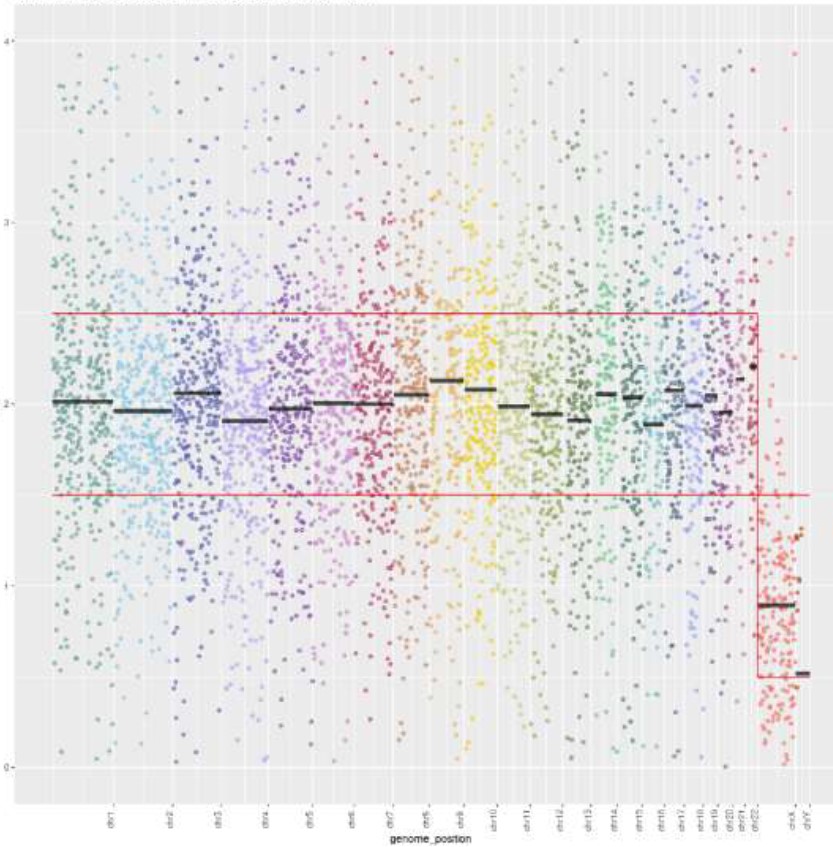
(a)
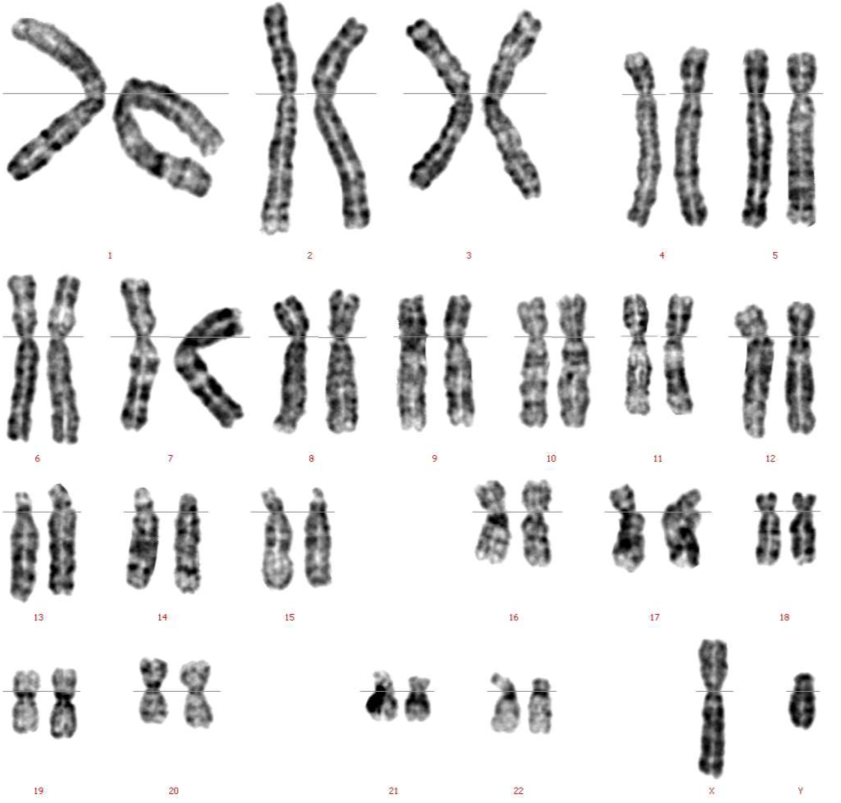
(b)
[1] Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016;18(10):1056-1065. http://doi.org/10.1038/gim.2016.97.
[2] Kalousek DK, Dill FJ. Chromosomal mosaicism confined to the placenta in human conceptions. Science 1983;221:665–7.
[3] Simoni G, Sirchia SM. Confined placental mosaicism. Prenat Diagn 1994;14:1185–9.
[4] Grati FR, Malvestiti F, Branca L, et al. Chromosomal mosaicism in the fetoplacental unit best practice & research. Clin Obstet Gynaecol 2017;42:39–52.
[5] Battaglia P, Baroncini A, Mattarozzi A, et al. Cytogenetic follow-up of chromosomal mosaicism detected in first-trimester prenatal diagnosis. Prenat Diagn 2014;34:739–47.
[6] Wilkins-Haug L, Quade B, Morton CC. Confined placental mosaicism as a risk factor among newborns with fetal growth restriction. Prenat Diagn 2006;26:428–32.
[7] Stipoljev F, Latin V, Kos M, Miskovic B, Kurjak A. Correlation of confined placental mosaicism with fetal intrauterine growth retardation. A case control study of placentas at delivery. Fetal Diagn Ther 2001;16:4–9.
[8] Kalousek DK, Howard-Peebles PN, Olson SB, et al. Confirmation of CVS mosaicism in term placentae and high frequency of intrauterine growth retardation association with confined placental mosaicism. Prenat Diagn 1991;11:743–50.
[9] Mardy A, Wapner RJ. Confined placental mosaicism and its impact on confirmation of NIPT results. Am J Med Genet C Semin Med Genet 2016;172:118–22.
[10] Brison N, Neofytou M, Dehaspe L, et al. Predicting fetoplacental chromosomal mosaicism during non-invasive prenatal testing. Prenat Diagn 2018;38:258–66.
[11] Toutain J, Goutte-Gattat D, Horovitz J, Saura R. Confined placental mosaicism revisited: Impact on pregnancy characteristics and outcome. PLoS One 2018;13:e0195905.