Manuscript
Introduction
Human reproduction constitutes a highly inefficient and selective phenomenon. Currently, even in optimal circumstances, the highest probability of achieving pregnancy is estimated at around 30-40 % per ovulatory cycle(1). The main cause of this inefficiency is due to the large proportion of generated aneuploid embryos, characteristic that correlates with clinical phenotypes, such as infertility and spontaneous abortion. This high prevalence of aneuploidies during pre-implantation also constitutes an important factor that contributes to the failure of assisted reproduction treatment (ART).
The preimplantation genetic test for chromosomal aneuploidies (PGT-A) has been transformed into an increasingly common practice in ART. PGT-A has widely shown its usefulness in advanced maternal age patients, implantation failure, recurring abortion, and previous pregnancies with chromosomopathies(2).
Although embryonic mosaicism has been known for decades, the greater precision and sensitivity of Next Generation Sequencing techniques (NGS) has provided the opportunity to identify more clearly an intermediate number of copies for a single chromosome(3), thus being able to detect a euploid-aneuploid mosaicism with greater frequency. It is currently considered that NGS can identify chromosomal abnormalities that are present in at least 20% of the cells (1 in 5)(5).
Embryonic mosaicism continues to represent a complex phenomenon that is considered by many authors as a limiting factor in the interpretation of PGT-A cycles results. In a mosaic embryo coexist at least 2 cell lines with different karyotypes, being able to coexist euploid and aneuploid cells or coexist only aneuploid cells with different anomalies.
Origin of mosaic embryos.
While completely aneuploid embryos originate from an aneuploid zygote, derivative from one or both aneuploid gametes, mosaic embryos rise from an euploid zygote that suffers some abnormal mitosis (Figure 1A). The earlier this error occurred, the aneuploidy can spread to a greater number of cells, giving rise to embryos with a high percentage of abnormal cells (high-level mosaicism; Figure 1B). The occurrence of these mitotic errors could be due to the characteristics of these first divisions: the use of components and materials of oocyte origin (prior to the activation of the embryonic genome), the presence of more permissive cell cycle control mechanisms, etc.(6)(7). Recently, some publications have shown that the first mitotic division is highly susceptible to errors(6), suggesting it may be responsible for the early appearance of mosaicism.
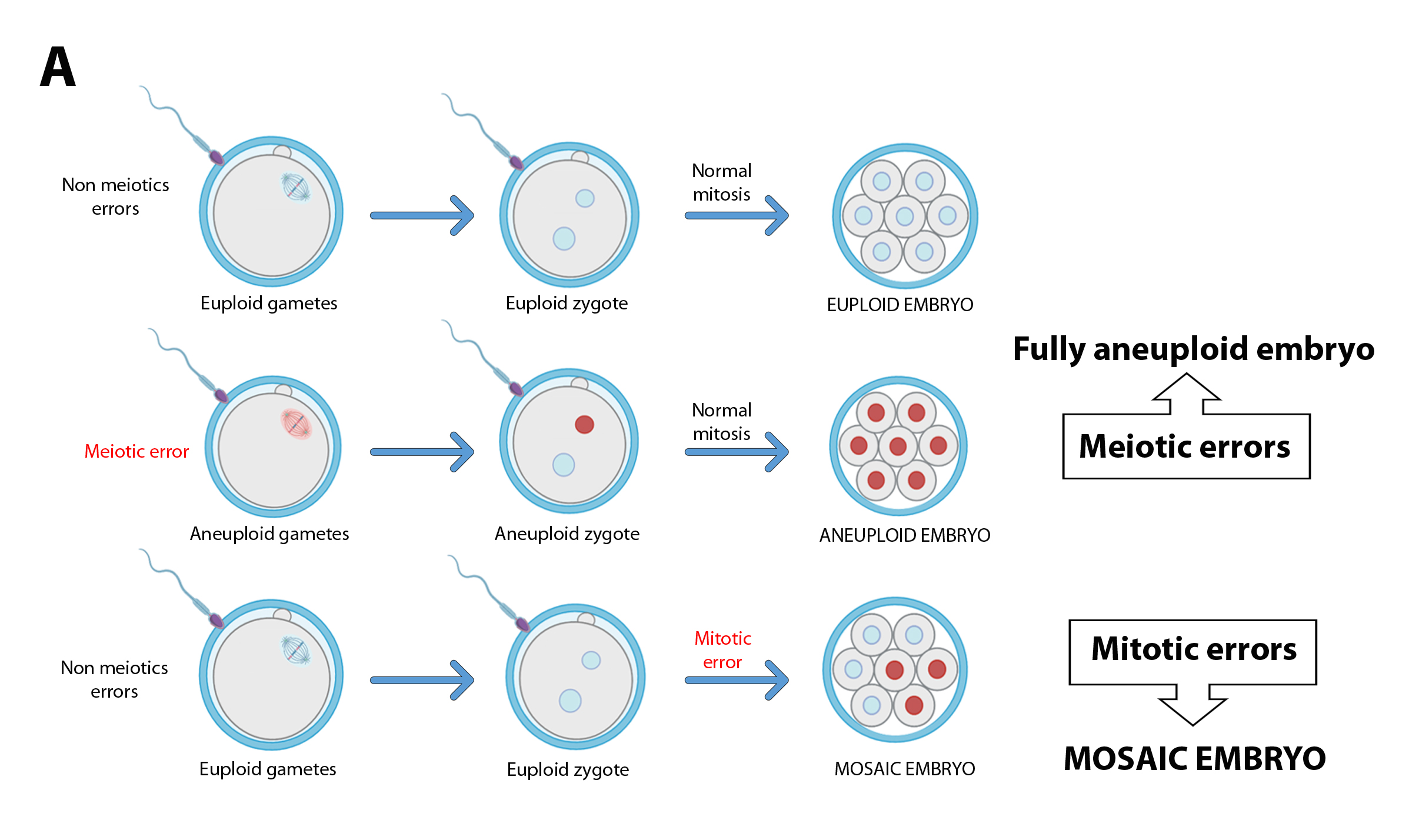
Figure 1A. While euploid embryos originate from aneuploid zygotes, mosaic embryos come from euploid zygotos that suffer errors during mitotic divisions during the early stages of preimplantation development.
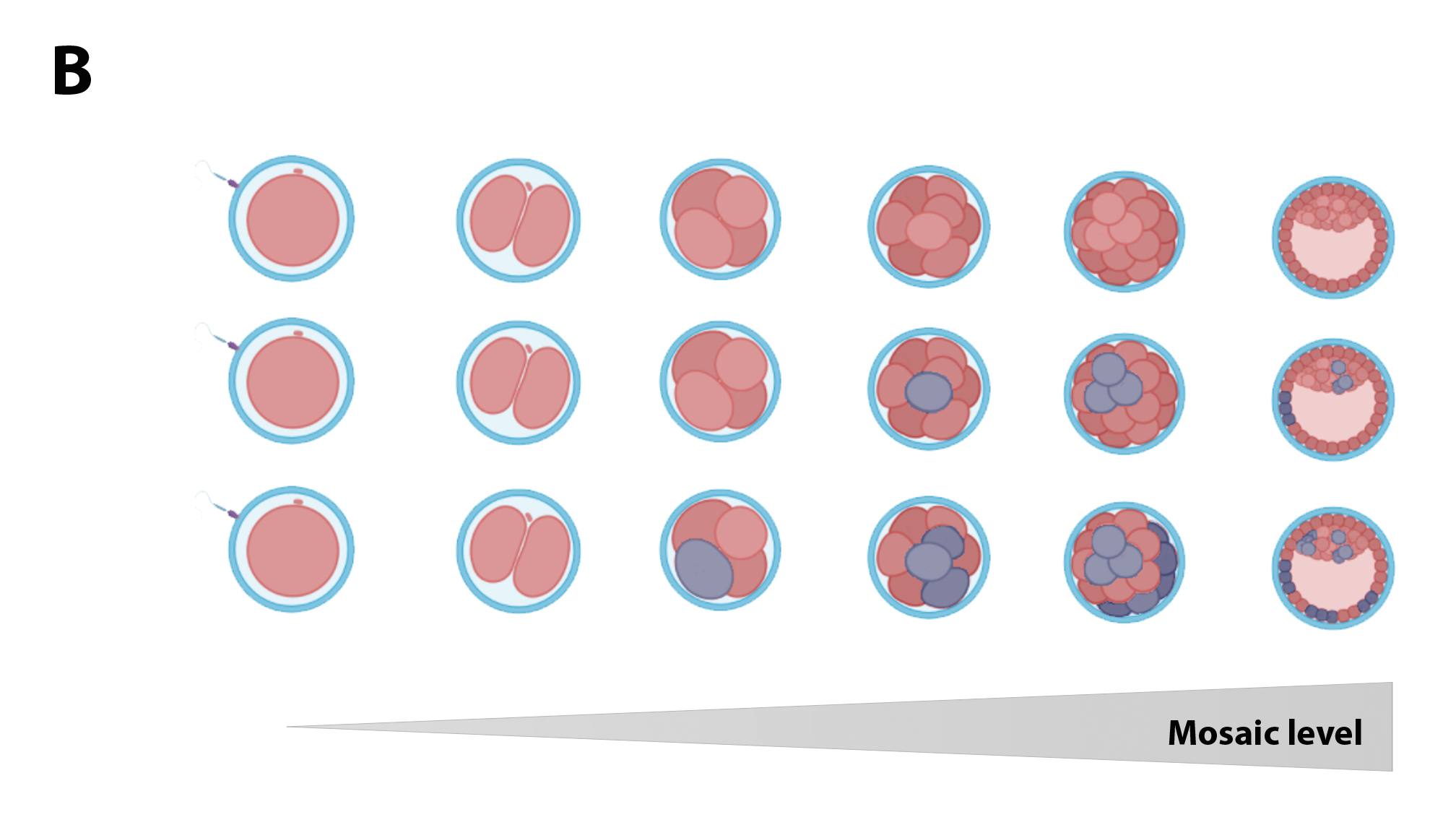
Figure 1B. The earlier the mitotic error occurs, the greater the number of aneuploid cells present in the embryo.
There are several cellular mechanisms that can cause errors in chromosomal segregation during embryonic divisions, mainly those that result in bad segregations of sister chromatids. Within them, anaphase lag is considered the major cause of mosaic embryos. This chromosome lagging, together with the possibility of abnormal tripolar spindle formation that result in a massive loss of chromosomes ("chaotic" mosaicism), have been described as the main errors during the first mitotic division(6).
Factors influencing the generation of mosaic embryos.
Few factors have been related to promoting embryonic mosaicism. Several publications have shown a greater tendency to produce mosaic embryos in couples with male factor, being even higher in the case of severe male factors and testicular sperm(9),(10),(11). Any disorder of the sperm centrosome can theoretically produce mosaicism in the embryo. Sperm aster formation has been shown to be delayed in infertile males compared to fertile male controls. This could cause delayed syngamy and subsequent cleavage, and possibly induce aneuploidy and mosaicism. On the other hand, cellular stress factors such as variations in pH, osmolality and temperature can negatively impact mitotic divisions, affecting correct chromosome segregation(12).
While the incidence of meiotic errors and embryonic aneuploidies is highly related to maternal age, mosaicism appears to be unrelated to either maternal or paternal age. However, there are some publications that suggest a slight decrease in the rates of embryonic mosaicism in patients older than 37 years(13),(14).
Incidence of embryo mosaicism.
Determining the incidence of embryonic mosaicism is a complex issue since its frequency varies considerably between clinics and analysis laboratories, depending on the detection technique, cells number and chromosomes analyzed, cells origin and thresholds established to define the levels of mosaicism (see Detection of mosaic embryos section). Instead, and as mentioned above, there are also certain biological factors related to culture conditions that can influence mosaicism levels.
According to the PGD International Society (PGDIS), the incidence of mosaicism reported at the blastocyst stage and using NGS methods varies between clinics, from 2% to 40%, although most clinics report levels between 5% and 15%(15). ASRM recognizes levels of mosaicism from 3% to 20% depending on the NGS platform and the analysis parameters used (16). CooperGenomis reports on its website a global incidence of 13.7% on more than 10,000 embryos analyzed(17). Mosaicism levels in pre-compaction embryos are much higher, clearly reflecting that the presence of a certain percentage of aneuploid cells can compromise embryonic development. Among the wide variety of strategies that can be used, the analysis of multifocal biopsies (usually one ICM biopsy and 4 biopsies from different areas of the TE) represents the most credible approach to corroborate the chromosome mosaicism, although clearly not be of clinical utility. Popovic et al., using this technique, determined a mosaicism level of 37% in the studied population. Similarly, recently, Ren et al.(18), using single cell sequencing, verified the presence of mosaicism in more than 60% of the analyzed embryos. As can be seen, there is great variability between the different laboratories, depending on the characteristics of the patients and the culture conditions of each place, but as will be seen later, it is mainly due to technical and methodological issues, related to diagnostic techniques.
In addition to this variability in the incidence of mosaicism, another factor that attracts a lot of attention is the great difference between the average levels of mosaic embryos detected and the values of mosaicism found in the products of conception (placenta/fetus). Placental mosacism has been reported in around 2% of the samples studied (similar values between natural conceptions and by in vitro fertilization(19)), and of these cases, the presence of fetus mosacism could be verified in only approximately 13%.
Mosaic embryo transfer.
As initially mentioned, the use of NGS in PGT-A cycles was characterized by a significant increase in the in mosaic embryos reported, generating a great uncertainty regarding the prevalence of mosaicism in human blastocysts, its biological importance, and above all, the destination that should be given to these embryos. The decision to transfer these embryos contemplates not only that the mosaicism found in TE becomes a confined placental mosaicism, with the risks that this entails (intrauterine growth restriction, placental insufficiency, etc.), but also that it may be present also in the fetus, compromising its health. Chromosomal mosaicism has been related to the presence of various diseases such as Alzheimer's and frontotemporal dementia(20).
In 2015, Greco et al. were the first to report the clinical results after transferring mosaic embryos(21). Currently, there are numerous publications that detail the results obtained after this kind of practice. In the publication by Treff et al.(22), a table is included detailing 25 highly relevant studies, in which the results obtained after transferring mosaic embryos are shown. Within these studies, the success rate is highly variable, depending on the number of embryos transferred and the goals set in each work (pregnancy rate, live birth, etc.). In this table we can also verify the birth of a single baby carrying the mosaicism initially detected in the embryo(23), after transferring 2759 mosaic embryos. After a pregnancy without any anomaly, a healthy baby was born at week 37, with no apparent phenotypic abnormalities, but with a peripheral blood karyotype showing chromosome 2 monosomy in 2% of cells studied. Beyond the characteristics of this specific case, the fact that it is the only pregnancy carrying a mosaicism among the 2759 transferred embryos represents a percentage of affected pregnancies well below the average value found even for embryo transfers without genetic analysis (0.04% (1/2759) versus 2%). This conclusion leads to doubts about the power of PGT-A to effectively detect mosaic embryos.
Among the studies listed in Treff's table, Viotti et al. reported the results of a multicenter study in which 1000 mosaic embryos were transferred(24). The authors reported lower clinical results than euploid embryos (implantation rates, ongoing pregnancy and live birth, miscarriage) especially if embryos with mosaicisms involving complete chromosomes rather than segmental abnormalities are transferred. The results were even worse against high level mosaicism and against abnormalities involving multiple chromosomes. Despite this, the general population of mosaic embryos presented an implantation and evolutionary pregnancy of 46.5% and 37.0%, respectively. This allows us to conclude that despite presenting inferior clinical results to euploid embryos, mosaic embryo transfer should be taken into account, given that in certain cases it can lead to the success of assisted fertilization procedures, and that not using them would be condemning the storage to potentially viable embryos, with some possibility of leading to the birth of a baby. It is also important to mention that in this study no mention is made about the phenotype of the babies born.
Like many other retrospective studies, the previous publication presents a great bias from the strictly scientific point of view: mosaic embryos are transferred only in the absence of euploid embryos, that is, in suboptimal cycles, even in cycles where the patients have already transferred normal embryos without positive results. In Capalbo's study, embryos that showed a low or moderate level of mosaicism were blindly informed as euploid without distinction of uniformly euploid embryos. In this way, embryos were selected to transfer only based on their morphology. Considering the three groups (euploid, low level mosaic and medium level mosaic), no significant difference was observed in clinical parameters (pregnancy rates, abortion and living births). In some miscarriage cases (4/52), the abortion material was cytogenetically evaluated, not being aneuploid. Babies born presented normal obstetric and neonatal parameters, and in cases where a peripheral blood karyotype was performed (38/386 of born babies), these were also normal(25).
These two articles, like many others, seem to indicate that the transfer of mosaic embryos (at least those that do not have very complex anomalies) would represent a relatively safe practice, despite presenting clinical results lower than euploid embryos. Recently, Viotti and col., compared pregnancies resulting from embryos classified as euploid or mosaic, finding that babies born of mosaic embryo transfers are like babies of euploid embryo transfers(26). On this basis, It must be considered that some potentially viable blastocysts are clinically classified as inappropriate to be transferred, negatively interfering with the result of an assisted reproduction procedure, especially in patients with few or no normal embryo. In this way, the idea of achieving conception products (placenta and/or fetus) free of anomalies despite transferring mosaic embryos, leads us to rethink real capacity of the PGT-A in reliably detecting embryonic mosaicism. To explain this phenomenon, we can travel deferens roads. One of these pathways is based on the existence of the so-called embryonic autocorrection phenomenon, which is based on the ability of an embryo, of being able to "eliminate" aneuploid cells.
There are different proposed mechanisms for this autocorrection to occur, which are not exclusive to each other, but could be acting synergistically to achieve greater correction. Embryonic mortality and clonal exhaustion models suggest that aneuploid cells do not survive and are lost during implementation. A study in mice was the first to show the progressive exhaustion of aneuploid cells and in providing direct evidence that apoptosis in ICM is a mechanism to eliminate aneuploid cells(27). In this study, the authors induced aneuploidies in early mouse embryos using reversine, then creating 8-cell chimeras embryos mixing aneuploid and control cells control. Embryos were cultivated to the blastocyst stage, checking a decrease in the percentage of aneuploid cells as a function of development time, due to an increase in apoptosis in the ICM. No variations were observed in the number of abnormal cells in the TE. On the other hand, chimeras embryos were created with different proportions of euploid and aneuploid cells. They verified that, if there is a certain relationship between euploid and aneuploid cells, lethality previously observed with aneuploidy embryos could be avoided, being able to achieve an evolutionary pregnancy.
Another strategy for this selective elimination of abnormal cells is the exclusion of these from the developing embryo. It has been proven that the exclusion of aneuploid cells in the morula stage could also act as a potential self-correction mechanism(28),(29). Many of excluded cells from euploid blastocysts are often aneuploids or with very fragmented DNA, and cells excluded from blastocysts with simple aneuploidies generally contain more complex aneuploidies and segmental aberrations(30).
Alternatively, the possibility that aneuploid cells are preferentially located in the TE has also been proposed. This hypothesis is controversial because, although there are studies that support this hypothesis(31), there are several publications that do not show a preferential distribution of aneuploid cells in the TE(32).
Finally, it is suggested that aneuploid cells can lead to diploid cells through chromosomes loss or gain (trisomic/monosomic rescue model; Figure 3). Some authors give little credibility to this model; however, the real presence of uniparental disomy (UPD, a consequence of this model) in natural embryonic suggests that it can occur, although in a very low rate: in a study of 3401 embryos, finding a general frequency of 0.06%(33). A corrected monosomy using this model would always result in UPD while trisomy correction would result in UPD a third of the time. However, correction events that do not result in uniparental homologous are also theoretically possible and can be underestimated since they are impossible to detect.
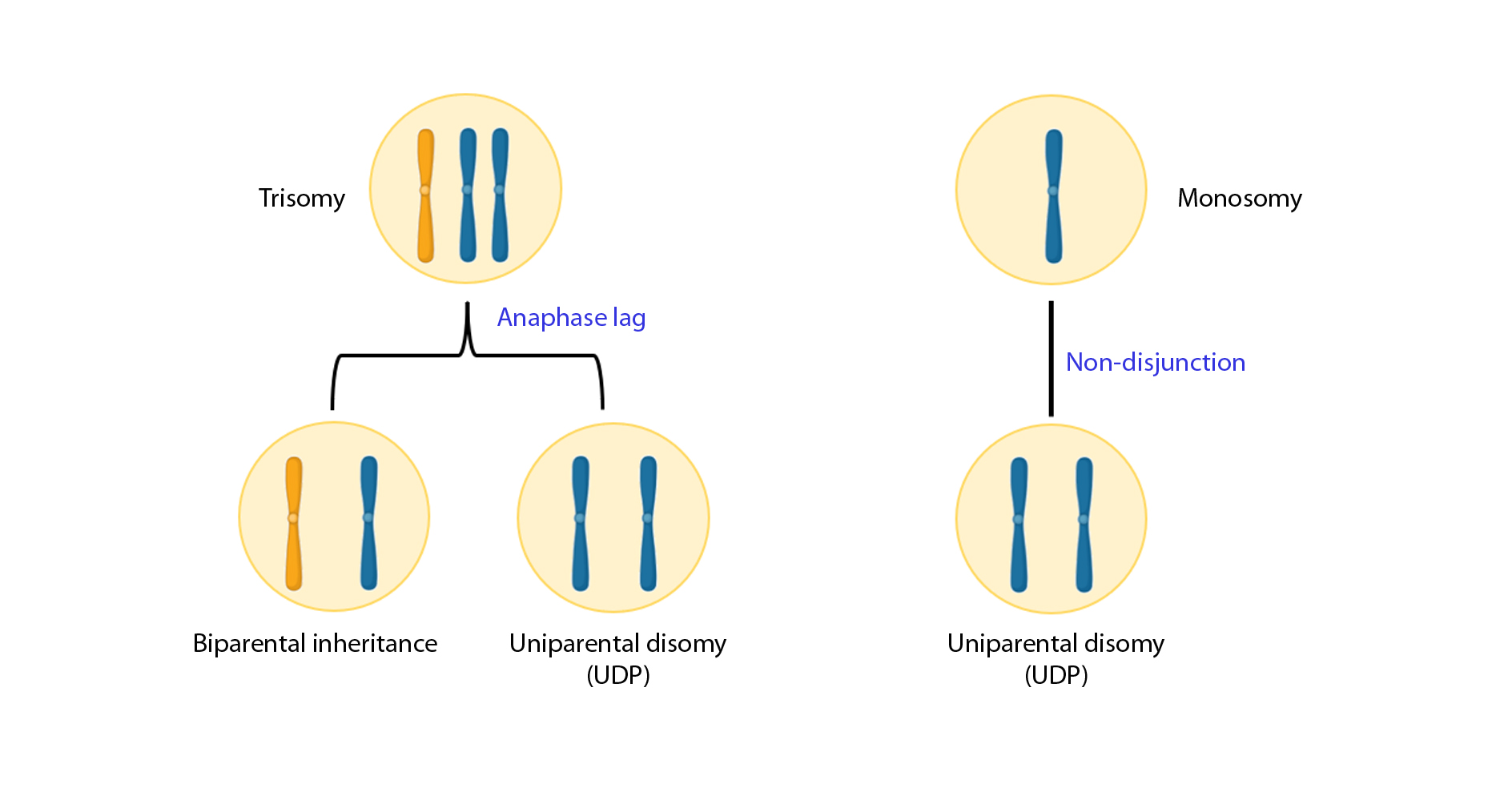
Figure 3. Aneuploid cells can lead to diploid cells through chromosomes loss or gain.
Despite the seriousness of the above publications (and of several not included) that show evidence of the existence of this mechanism of embryonic rescue, justify the results obtained by transferring thousands of mosaic embryos, in the absence of affected conception products, it would be overestimating the influence of these mechanisms. For this reason, several authors indicate that the absence of affected conception products is simply because these embryos were not effectively mosaic embryos, then being false positives. This reasoning is developed in Nathan Treff's paper where the birth of a single baby affected after the transfer of more than 2500 mosaic embryos is mentioned, giving an incidence of affected births of approximately 0.04%(22), a value much less than 2% incidence detected in natural conceptions. This big difference cannot be explained solely by self-correction mechanisms.
The existence of these false positives should not be denied and is strongly related to the diagnosis of embryonic mosaicism. A systematic review where the results of the reanalysis of 289 embryos previously diagnosed as mosaic were linked to a single tea biopsy, could corroborate this result in only 42% of cases; The remaining embryos were diagnosed as euploids and aneuploidies (29% and 28% respectively)(35). The transfer of these really aneuploid embryos, but considered mosaics, would be justifying (at least part) the lower clinical results, compared to the transfer of real euploid embryos.
These results clearly show how the correct diagnosis of a mosaic embryo remains a subject of continuous debate, mainly due to the doubts that are still presented about the capacity of the profiles with intermediate number of copies of a chromosome, to be able to correctly predict the presence of a true mosaic embryo.
Understanding the reason why an embryo can be diagnosed as mosaic, necessarily includes evaluating the factors involved and affecting its detection.
Mosaic embryo detection.
From the point of view of the diagnosis, a mosaic embryo has an intermediate value of copies of a chromosome between monosomy/disomy or trisomy/disomy(15). The diagnosis of mosaicism is highly complex and can be influenced by several methodological variables (Table 1).
| Biopsy-associated factors |
NGS-associated factors |
| Biopsy technique |
Amplification protocols (Whole genome amplification) |
| Biopsied cell quality |
Platform specificity and sensitivity |
| Cell number |
Thresholds or limits for diagnosis |
Table 1.
We must start considering the analysis material, the TE biopsy. It is recommended that biopsies include between 5 and 10 integral cells. A lower number of cells could exclude euploid/aneuploid cells that would be defining mosaicism, while a larger number of cells could compromise embryo viability(36). In addition, the simple fact that the diagnosis is based on the analysis of a single biopsy may be underestimating the phenomenon of embryonic mosaicism, since, as has been demonstrated in several publications, the distribution of abnormal cells is not uniform sowing within embryonic TE.
The state of biopsy cells is of great relevance, since the presence of cells with compromised integrity can lead to the loss or deterioration of the chromosomal material, conditioning the analysis. For this reason, different factors referring to the biopsy that must be taken to maintain the integrity of the cells and their chromosomal content must be considered. For example, the excessive use of the laser, the temperature and the time in the tubing, the washing conditions before the tubing, the shipping conditions to the molecular laboratory, are some of them.
The basic concept of a biopsy represents a limiting factor for the detection of mosaic embryos: on the one hand, biopsy can include only euploid cells (or aneuploids), leaving aside a population of aneuploid cells (euploids) located in another sector of the sector of the TE (Figure 2A), or may include cells with complementary aneuploidies that as a whole would complete the disomy; in both cases, embryonic mosaicism would be masked by a technical artifact (Figure 2B).
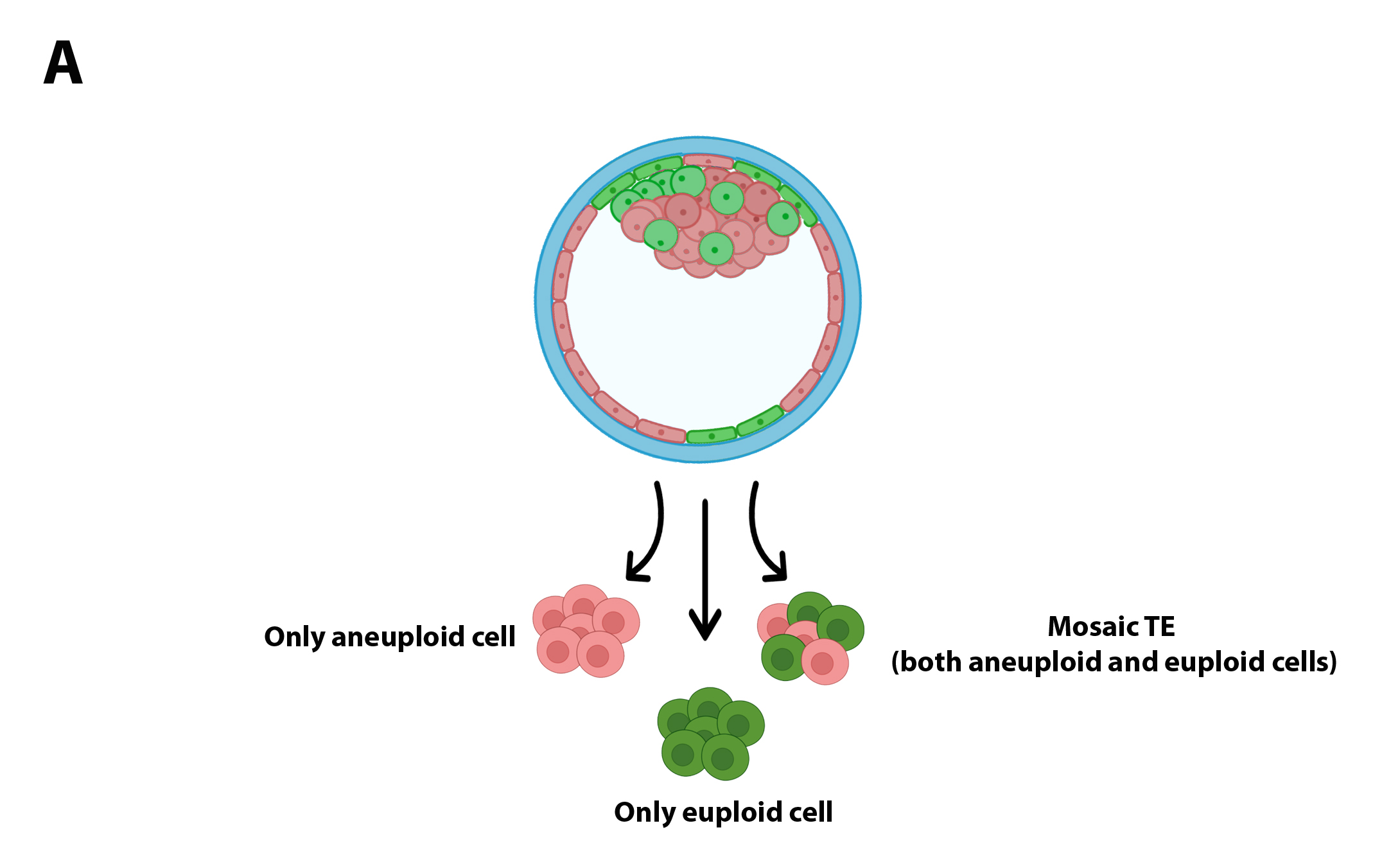
Figure 2A.
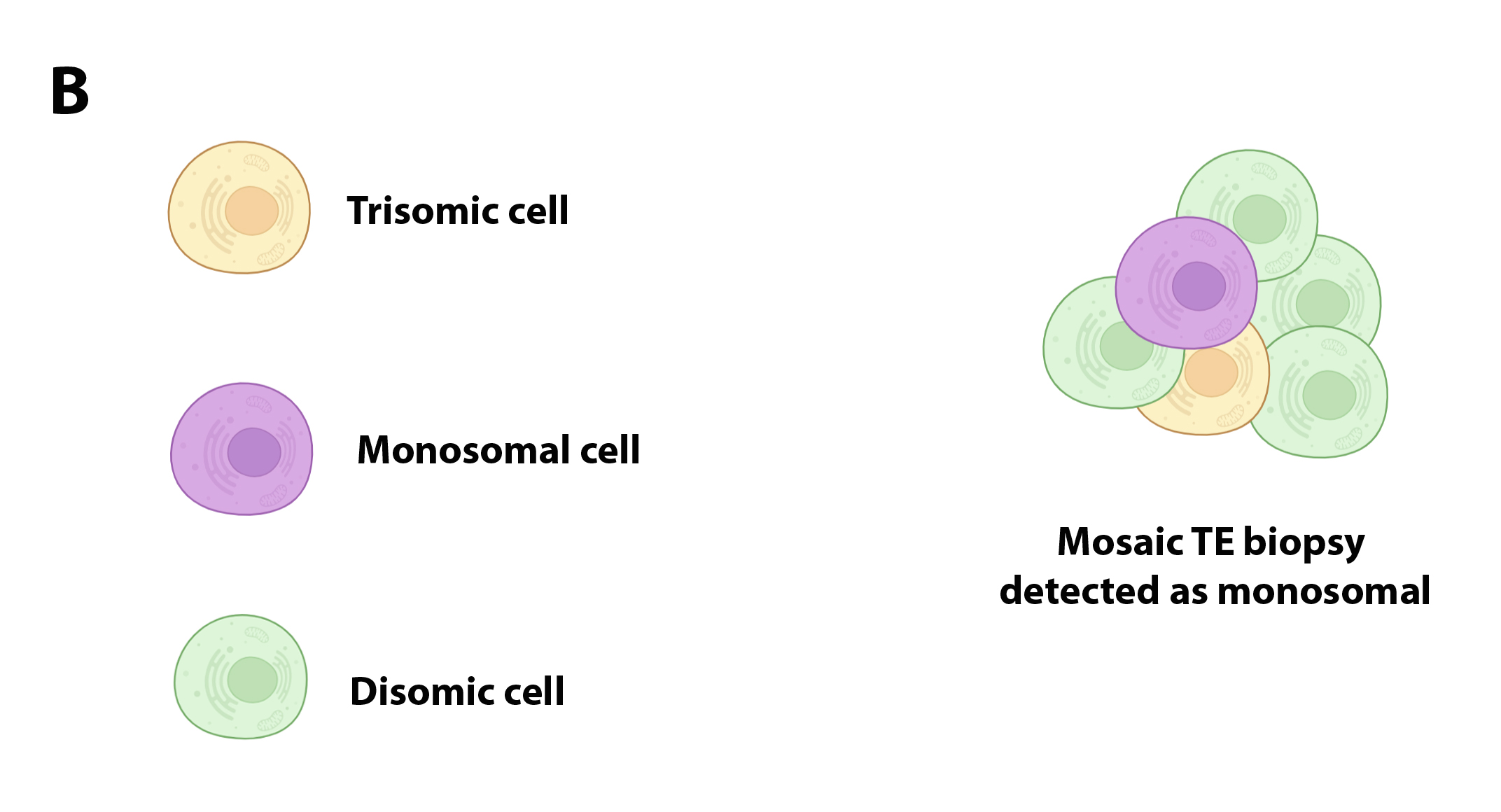
Figure 2B.
The characteristics of the biopsy of you can influence the result of the chromosomal diagnosis.
As is known, whole genome amplification (WGA) allows transforming the small amounts of DNA obtained by the embryonic biopsy, in quantities that can be analyzed by modern molecular techniques. However, WGA methods can lead to inefficient amplification, which leads to insufficient or excessive representation of some part of the genome(22). The most modern WGA protocols, such as those based on MALBAC technology, tries to minimize these errors.
Another important factor to evaluate is the concept of "thresholds". Most laboratories use one of the following criteria to consider an embryo as mosaic and establish whether the embryo carries a "high" or "low" level mosaicism. Some laboratories consider an embryo as mosaic when TE biopsy evaluated contains between 30% -70% of aneuploid cells, outside those limits, the embryo is considered as euploid or aneuploid. In this way, it is also defined as an embryo with “low level” mosaicism when it has 30% -50% of aneuploid cells, while it is considered as a mosaic embryo of “high level” when biopsy contains 50%-70% of aneuploid cells. Other laboratories use a less strict criterion of 20%-80%, establishing low- and high-level mosaicism between 20%-40% and 40%-80%. The choice of one of these (or some other) thresholds, has direct influence on the diagnosis. Less strict limits (20%-80%) increase sensitivity to detect mosaic embryos but with decrease in detection specificity since it increases the percentage of false positives(37).
NGS platforms also have must be evaluated and validated to determine their ability to detect embryonic mosaicisms. Classically, platforms have been validated using artificial mixtures of euploid cells and aneuploids, a strategy that is far from what happens with a TE biopsy. The platforms must have a specificity that allows it to clearly distinguish the biological signals product of the detection of a certain sequence, of the technical noise of the equipment.
Although what is expressed until now is a brief description of the technical inconveniences that surround the diagnosis of embryonic mosaicism, they are enough to highlight the complexity of the detection of a mosaic embryo.
The need to optimize the diagnostic and results interpretation methods in order to have a reliable determination of embryonic mosaicism, is clear and accepted. In this regard, recently Buldo-Licciardi published a study that uses artificial intelligence (AI) for the correct interpretation of NGS results, with the aim of reducing human subjectivity in the interpretation of the results, improving the sensitivity and specificity of the diagnosis. Using this platform, a more precise diagnosis was achieved that positively impacted the clinical results(38).
Final considerations.
From all the above we can affirm that:
- The diagnosis of embryonic mosaicism constitutes a challenge of great complexity, characterized by the different methodological challenges, which must be considered to avoid falling into false positives.
- The experience developed so far would seem to indicate that mosaic embryos transfer would be a relatively safe practice, since it does not correlate with a high probability of generating of conception affected.
- The clinical results obtained when transferring mosaic embryos are more unfavorable than those obtained by transferring fully euploid embryos, especially as the complexity of the chromosomal abnormalities present increased.
Based on these statements, we would not have to question whether it is possible to transfer a mosaic embryo, but we should reflect on what mosaic embryo we can transfer. To collaborate with these decisions, various scientific societies have provided some guides in this regard(15),(16),(39). While each statement has its own premises, they all agree in some characteristics in common:
- Mosaic embryos should be transferred only in the absence of available euploid embryos.
- Couples should take knowledge of everything that implies the transfer of these embryos and provide their consent.
- Each society has different guides to prioritize the transfer of a mosaic embryo over others, based on the chromosomes involved and the level of mosaicism, mainly.
In 2018, Grati and collaborators published a study on samples of chorionic villi and products of conception. The authors developed a practical guide that evaluates the real risk of transferring a mosaic embryo, evaluating the phenotypes detected in natural conceptions, and based on chromosomal anomalies(40).
There are many publications that support the decision to transfer mosaic embryos, evaluating the relevant risks, as well as the specific characteristics of embryo anomaly. This decision should be taken by health professionals with patients. A couple must know, before starting a cycle of PGT-A, all the advantages and limitations of this technique, including the possibility that some of their embryos be diagnosed as mosaic. They must be informed about the incidence of this phenomenon, the considerations regarding their diagnosis, and the possible risks they imply deciding transfer them. They must know that the transfer of embryos in mosaic is associated with lower implantation rates and a greater risk of spontaneous abortion, than when transferring euploid embryos. It is also important to mention that, although so far more than 100 births have been reported by transfer of embryos in mosaic, without abnormal phenotype, greater long -term studies are necessary to define their true security, given that almost all these babies do not reach 10 years of age.
The existence of large incidence of false positives in relation to the embryonic diagnosis is clearly affecting the decision related to the transfer of a true mosaic embryo. On the other hand, the results obtained by multifocal biopsies and individual cell sequencing(1),(18), as well as studies that indicate the large rate of mitotic errors during the first preimplantation divisions(6), seem to indicate that real mosaicism rate is superior even to the estimated. However, there would be different biological mechanisms that could ensure the development of a healthy pregnancy, eliminating abnormal cells, if the number of normal cells is sufficient(28).
In normal tissues, alterations of the number of copies of chromosomes that range between 1.1% and 10.6% are detected, without compromising its functionality(41). The appearance of an aneuploidy induces complex cellular responses that affect cell destination. Convincing evidence of cells in culture suggests that the appearance of aneuploidies is associated with the activation of roads that mediate cell stress, which can reduce the proliferative capacity of these cells. When these cells can counteract these effects, they have great risks of transforming into tumor cells(42). In addition to cancer, the appearance of aneuploid cells has been linked to other physiological processes, such as development and aging. In fact, in some tissues, such as brain and liver, the appearance of aneuploid cells seems beneficial(43). From this point of view, the existence of a few aneuploid cells within the approximately 200 cells that form a blastocyst (as in the case of low -level mosaicisms), may not be compromising the healthy course of an eventual pregnancy and birth.
We have advanced a lot in the understanding of embryonic mosaicism, and perhaps, over time we realize that we have been overestimated this phenomenon.







