Manuscript
Introduction
Mitochondria are found in eukaryotic cells, with their most important function being their role in producing adenosine triphosphate (ATP). Mitochondria are present in most cells of the body, generally in numbers between 100-1000(1) and carrying 2-10 copies of mitochondrial DNA (mtDNA)(2). While the function and activity of mitochondria vary based on cell type, mitochondria generally play a role in oxidative phosphorylation, metabolic balance, cellular senescence, and maintenance of apoptotic mechanisms(3). Damage to mtDNA can result in mutations that can lead to many mitochondrial disorders. In particular, heteroplasmy in the tissues and organs of the fetus during fetal development can lead to the formation of mtDNAs with different mutant ratios and hence a qualitative transmission to the next generation.
In some cases, although the mitochondria in a mature oocyte (metaphase II) show a morphologically homogeneous structure, they may exhibit different effects depending on the level of mitochondrial polarity. Mitochondrial polarity refers to the electrical charge on the inner membrane, which is directly related to ATP energy production. A mitochondrion with a high membrane potential has a high rate of ATP production and plays an essential role in regulating CA++ hemostasis(4). Mitochondria undergo changes during fertilization, embryo development, and preimplantation. During metaphase I and II in the oocyte, mitochondria are homogeneously distributed throughout the cytoplasm, and an abnormal distribution of this homogeneous distribution through a network of microtubules could be detrimental to the fertilization of the oocyte and the developmental properties of the embryo. Therefore, structural changes in mitochondria affect the quality, potential, and development of the oocyte.
The correct mitochondrial functioning required for suitable oocyte development and embryonic development after the fertilization. Most probably intra cellular energy transferring may interrupt and it directly affect mitochondrial disfunction. Mitochondrial disfunction will bring limitation for ATP energy transport and it may give interruption to morph kinetic development of embryo right after cleavage stage. Mitochondrial dysfunction can be led by lack of energy or morphological abnormalities on the mitochondria. Both ways may create limitation for embryonic development and oocyte ageing in advance maternal age.
Mitochondrial Diseases and Mutations
PGD has been used in different ways, particularly to determine the number of mitochondria that may carry the mutation as the fetus grows. This technique aims to transfer embryos with a low risk of mutant mtDNA on a 6-10 cell basis in trophectoderm biopsy samples obtained from embryos obtained using the IVF technique. However, PGD technology is limited in its ability to provide results only for mitochondrial mutations and diseases caused by nuclear DNA. Mutations in mitochondrial DNA increase the risk of affecting embryos due to maternal involvement of mitochondria. Due to the lack of techniques particularly for estimating the degree of heteroplasmy in mtDNA, it is not easy to predict the risks associated with mtDNA(5). In this regard, mitochondrial transfer techniques have been successful in ensuring a healthy subsequent live birth in generations where mtDNA mutations have been detected.
Aging Oocyte
Mitochondria have a crucial role in the process of aging and metabolic decline, especially as a major source of reactive oxygen species. Increased levels of reactive oxygen species result in less mitochondrial energy being metabolically available (Figure 1). As age progresses, molecular senescence progresses to an advanced stage as increased levels of ROS affect mtDNA genome proliferation and both the morphological capacity and metabolic capacity of the oocyte are reduced(6). The increased level of aneuploidy, in particular, can be explained by the fact that, as meiosis is reactivated, a greater amount of intracellular mitochondrial energy will be needed, and this ATP energy is lower in older patients.
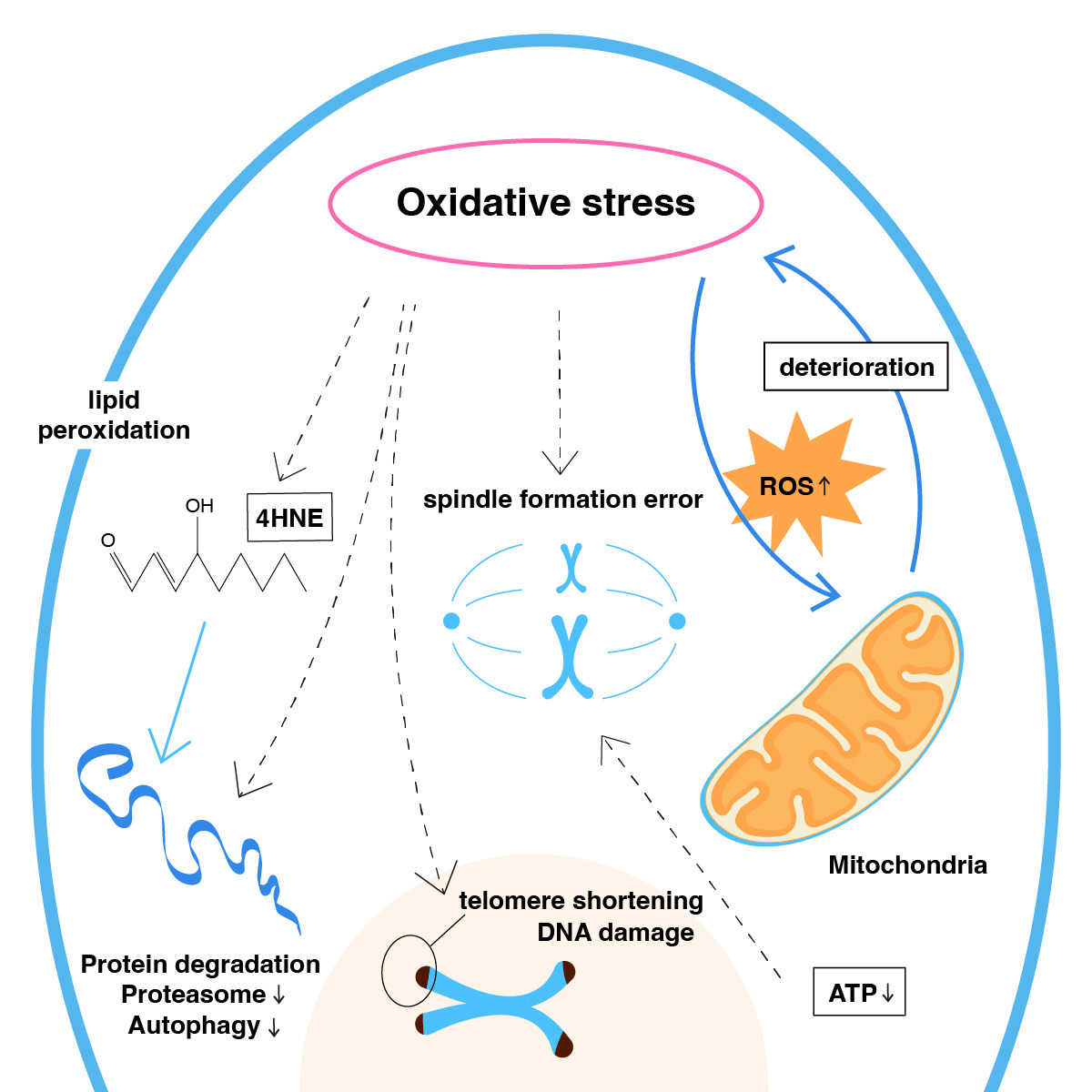
Figure 1. Mitochondria aging by oxidative stress.
Advanced Maternal Age and Mitochondria
Recent changes in living conditions, changes in women's social status, and postponement of childbearing due to career and occupational priorities have created the problem of achieving pregnancy at advanced reproductive age. The recognition that mitochondria are the center of quality power, especially in the oocyte cell, has confirmed that mitochondria play an important role in the problem of embryonic development at advanced age. In the normal process, paternal mitochondria are captured by autophagosomes and transferred to lysosomes for degradation after fertilization. Here, due to aging, mitochondria begin to show morphological changes and impaired activity. The loss of mitochondrial energetic activity at an older age has been associated with problems in the transition of embryos from cleavage to blastocyst at the cleavage stage of embryo development when energy requirements are high. The use of young mitochondria in these patients has been shown to significantly increase the rate of blastocysts obtained from embryos. In oocyte donation, it is observed that embryos obtained from oocytes obtained from young donors can easily achieve pregnancy when transferred to elderly patients. The most significant finding here is the high rate of euploid embryos in young patients, which leads to a higher chance of pregnancy success. Additionally, it has been observed that young oocytes had a high euploid rate, and euploid embryos were obtained from euploid oocytes. In both mouse and other animal experiments, it has been found that older oocytes had both a very low euploid rate and very low mitochondrial activity. It has also been noted that older women have much lower mtDNA at the zygote stage than younger women and that embryos from these zygotes do not reach the cleavage stage to the blastocyst stage.
Mitochondrial Regulation in Oocyte Development and Function
Results from different studies have proven that pyruvate, rather than glucose, is necessary for the maturation and maintenance of the oocyte. In this regard, the active use of pyruvate and the maintenance of the oocyte depends on the amount of mitochondrial activity. The maturation and maintenance of the oocyte are ensured by the production of ATP by the mitochondria and the beta-oxidation of fatty acids(7). The dynamic structure of the mitochondria splits in two with the formation of fusion and shows cellular distribution, including in cumulus cells. During this fragmentation, almost all of the asymmetrical mitochondria remain inside the cell. All this process takes place in Meiosis I, while the mitochondria are completely incorporated into the cytoplasm in Meiosis II. The maximum level of ATP generated during this process, together with the Ca++ pathway through the Endoplasmic Reticulum, utilizes this energy for cellular development and embryo maintenance. The most critical situation here is the transmission of heteroplasmy between generations. Heteroplasmy represents more than one mitochondrial DNA variant present in the cell and each individual carry one. The degree to which a person carries the disease based on heteroplasmy and its intergenerational transmission also indicates the extent of the mitochondrial mutation(8).
Mitochondrial Donation
Using a fertility-proven donor oocyte with healthy mitochondria, especially in diseases related to mitochondrial mutations, eliminates the risks and ensures the formation of healthy embryos. For various reasons, many patients with mitochondrial disease are diagnosed late, and due to the advanced age at which treatment is started, both the quality and quantity of oocytes are limited. Naturally, blastocyst development is very limited in patients with this advanced age characteristic. With the identification of good embryo development in these elderly patients, it was realized that mitochondrial transfer may be an alternative to oocyte donation in elderly patients. This method utilizes the mitochondrial potential of high-quality donor oocytes to produce high ATP energy, resulting in high-quality blastocysts in many patients. However, progress in correcting chromosomal defects in embryos obtained by this method is limited.
Meiotic Spindle Transfer
The meiotic spindle is the structure that forms during anaphase between meiosis 1 and meiosis 2, and it also serves to transport centrosome proteins. The meiotic spindle has a fundamental role in the development and genetic structure of the oocyte. The formation of the meiotic spindle by the separation of the chromosome package in the first anaphase phase indicates that the transfer of chromosomal structure occurs in the later stages of embryonic development. Therefore, it can be hypothesized that the transfer of the meiotic spindle from the oocytes of older women to the young donor oocyte results in a much better morpho-kinetic development with higher mitochondrial energy and even a lower aneuploidy rate.
Oocytes from an older patient and young donor stimulated with the same trigger timing should be prepared for transfer by determining the location and size of the spindle using spindle imaging software and polarizer, if the oocyte is mature, immediately after separation of the cumulus complex structure. The 24-hour prior addition of Cytochalasin B to the incubated single-step culture medium protects the zona permeability and cytoplasmic structure of the oocyte during micromanipulation. The spindle is removed using spindle view technology with the help of a small transitional zone to be opened using the laser hatching method in the zone of the oocytes taken from both the elderly patient and the oocyte donor, and the spindle is removed and subjected to fusion in the HVJ-E virus (Figure 2). This process aims to eliminate DNA fragments from the cytoplasmic structure around the spindle and to transfer the spindle into the donor oocyte as a compact DNA carrier. Instead of the spindle removed from the donor oocyte, the spindle structure of the older patient is transferred similarly, and the oocyte is incubated in the prepared solution for a while to ensure the formation of a complete karyoplast. After this procedure, the oocytes are incubated for 1 hour, and then fertilization is performed using the piezzo ICSI procedure.
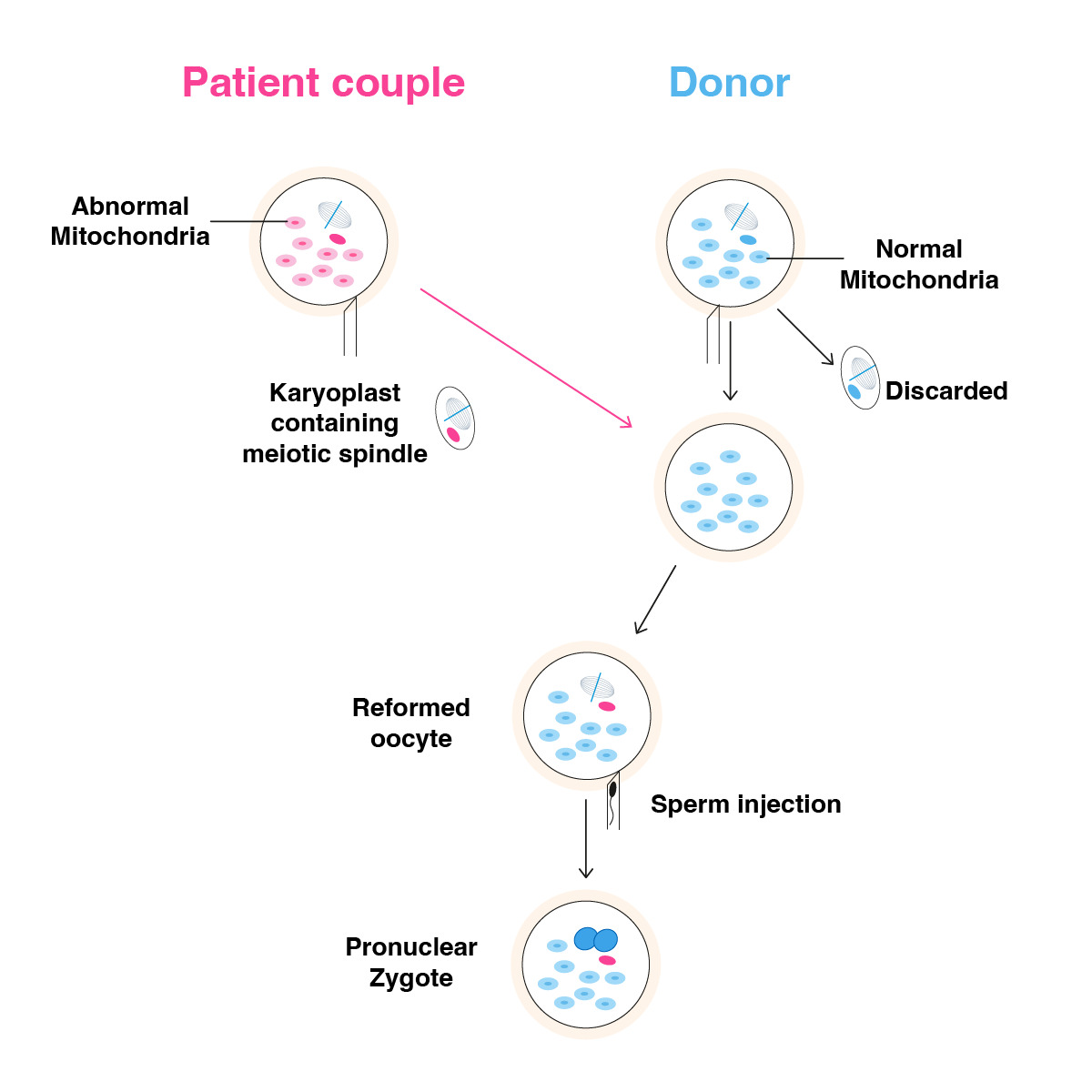
Figure 2. Meiotic spindle transfer.
Pronuclear Transfer
In patients of advanced maternal age, pronuclear transfer can be used as an alternative to spindle transfer, especially in the absence of a history of fertilization issues. The pronuclear transfer would be a good alternative, especially when oocyte quality is poor, and meiotic spindle structure cannot be observed. Another advantage of the pronuclear transfer procedure is the possibility of working on frozen zygote samples. Here, the zygote of an elderly patient can be used frozen, whereas a fresh zygote with fresh donor oocyte collection and a fresh fertilization process needs to be preferred for micromanipulation. Particularly after the ICSI procedure, early pronucleus detection ensures a better quality and successful micromanipulation procedure. Nocodozale and Cytothalasine B are added to the single-step culture medium incubated 24 hours before the procedure to preserve the zona permeability and cytoplasmic structures of donor and elderly patient zygotes in the petri dish. A crossing point is created using laser hatching on the zona of the zygotes, and with the help of a biopsy pipette, nuclei are extracted from both zygotes and introduced into the HVJ-E virus for fusion. The main purpose of this procedure is to transfer the compact DNA structure from the nucleus into the donor zygote by neutralizing the cytoplasmic fragments around the nuclei. After the transfer, the zygotes are left for a certain period to complete the karyoplast process and then transferred into the single-step culture medium, and embryo development is monitored (Figure 3).
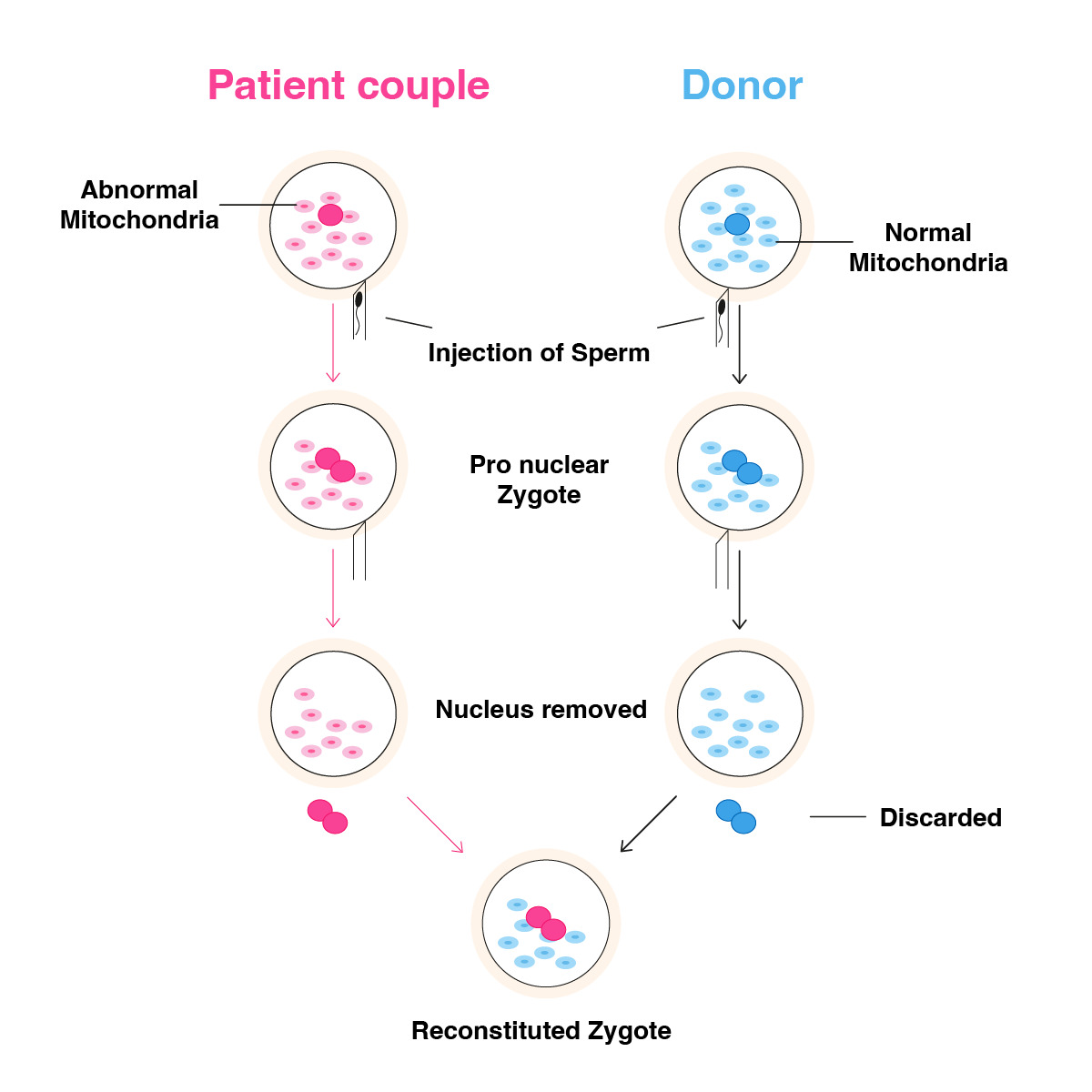
Figure 3. Pronuclear transfer.
Material and Methods
A total of 30 patients with no blastocyst development or total fertilization failure were included in the evaluation. Patients selected whom have minimum 5 IVF trials by ICSI technique with no receiving any blastocyst stage embryo. Patients selected with different AMH levels as between 0.7-2.0. All oocytes collected post trigger 35 hour and all oocytes fertilized after 3 hours from egg collection process and only ICSI technique used for fertilization. Embryo culturing process done by single step culture media and all embryo transfer done after PGT-A testing as frozen embryo transfer. All donor oocytes and zygotes used as fresh and spinal evaluated prior fertilization and mitochondrial transfer. The patients underwent pronuclear and meiotic spindle transfer procedures, embryo development was monitored, and chromosomal evaluation of the embryos was performed using the PGT-A technique. All donors selected for the procedure were fertility proven donors. Donors with at least 1 healthy child and at least 1 live birth in the oocyte donation procedure were determined and included in the procedure. A total of 297 oocytes from all patients were subjected to mitochondrial transfer. There was no loss of oocytes or zygotes after micromanipulation. No degeneration or morphologic damage was detected. The mean age of the patients who underwent the procedure was 39.4 years.
Result
A total of 29 patients elected to perform pronuclear transfer using healthy oocytes from donors, including 137 oocytes. After the procedure, 120 oocytes were fertilized (fertilization rate 87.6%).
When considering all 29 patients, and in terms of embryo development, 113 of the 120 fertilized oocytes progressed to the cleavage stage and 59 progressed to the blastocyst stage on day 5 or 6 (blastocyst formation rate 49.2%). All 59 of these blastocysts were biopsied for PGT-A, with 41 testing as euploid (euploidy rate 69.5%). A single euploid was transferred to each of the 29 patients, leading to 14 clinical pregnancies (clinical pregnancy rate 48.3%) and 14 live births (live birth rate 48.3%), without any pregnancy losses (Figure 4).
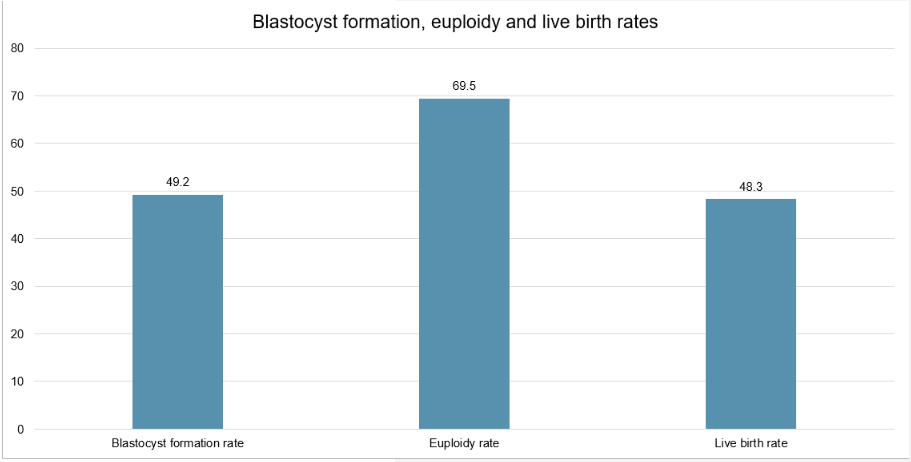
Figure 4. Blastocyst formation, euploidy and live birth rates.
The 29 patients were grouped based on their age, as <35 (n=2), 35-27 (n=6), 38-40 (n=9), 41-42 (n=6) and >42 (n=6). Based on these age groups, blastocyst formation rates were 47.8%, 50.0%, 45.0%, 50.0% and 66.7%, respectively; euploidy rates were 63.6%, 61.1%, 66.7%, 100.0% and 83.3%, respectively; clinical pregnancy and live birth rates were 100.0%, 50.0%, 44.4%, 50.0% and 33.3%, respectively (Table 1).
|
Blastocyst formation rate |
Euploidy rate |
Live birth rate |
| <35 (n=2) |
47.80% |
63.60% |
100.00% |
| 35-37 (n=6) |
50.00% |
61.10% |
50.00% |
| 38-40 (n=9) |
45.00% |
66.70% |
44.40% |
| 41-42 (n=6) |
50.00% |
100.00% |
50.00% |
>42 (n=6) |
66.70% |
83.30% |
33.30% |
Table 1. Blastocyst formation, euploidy and live birth rates according to age groups.
Although we recommend genetic testing for mitochondrial mutations for all babies born after pronuclear transfer, only 3 of the patients shared their results, which showed no abnormalities.
The fertilization rate after micromanipulation was 92.4%. While 6 of the patients had total fertilization failure previously, the fertilization rate obtained from 59 oocytes obtained from these patients was 89.25%. Regarding the cleavage development of the embryos, the development rate was determined as 85.74% based on the 3rd day of development. Based on 5th-day and 6th-day blastocyst development rates, only blastocysts with AA, BA, and BB quality were evaluated using the Gardner Scoring Method, and all blastocysts were evaluated with the PGD-A technique by performing trophectoderm biopsy procedures. On the 5th and 6th day of blastocyst development, an average blastocyst rate of 60.28% was obtained. The average rate of euploid blastocysts obtained after PGT-A was 60.68%. All patients underwent frozen embryo transfer. Single blastocyst transfer was performed in all patients, and the clinical pregnancy rate was 46.97%. Of these patients, 13 had a live birth, and the live birth rate was 48.28%. No genetic or morphologic abnormalities have been detected in babies born after live birth.
Discussion
This study aimed to report on blastocyst formation and euploidy rates of patients undergoing pronuclear transfer, demonstrating a 49.2% blastocyst formation rate and 69.5% euploidy rate.
All these patients failed to produce blastocysts after a minimum of 6 previous IVF cycles. The large increase in blastocyst formation rates seen in these patients is likely due to the donor mitochondria providing sufficient energy to power multiple cell divisions.
Six of these 29 patients in this study had previous IVF cycles with total fertilization failure, and after pronuclear transfer, the fertilization rate for these patients increased to 79.3% (based on 29 oocytes).
We performed pronuclear transfer on 29 patients of varying age, with most patients aged 38 and over, all of which demonstrated a high proportion of euploid embryos after PGT-A.
According to a recent report on the distribution of PGT-A results by age, the proportion of biopsied embryos that are euploid declines with advancing age, from 57.7% in patients aged <35 to 16.4% in patients over the age of 42. Our study demonstrates that reconstituted zygotes by pronuclear transfer show a high euploidy rate regardless of age. Although the sample size is small, of the 6 blastocysts generated in patients >42, 5 of them were euploid. This gives a euploidy rate of 83.3%, which is much higher than what would be expected at this age.
To the best of our knowledge, this is the first time that it's been shown that blastocysts by pronuclear transfer have high euploidy rates, irrespective of age. This is likely due to the highly functional donor mitochondria providing sufficient energy.
There are several limitations in this study, including the retrospective design and small sample size. We were also unable to collect more information on the 14 children born.
All clinical treatment methods are open to criticism and are based on a full assessment of the risks involved. Mitochondrial transfer should be considered with the main objective of eliminating the risks associated with mitochondrial diseases. Diseases due to mitochondrial mutations are mostly seen in elderly patients. Mitochondrial transfer has shown good results in problematic embryo development in diagnosed patients who want to have a healthy child at an advanced age, suggesting that this technique can be used in elderly patients. The healthier children born through this method can lead to healthier families and a healthier social structure. In the case of older patients, the ethical imperative is to provide an alternative to oocyte donation, based on the fact that each individual has the right to carry his or her own genetic material or DNA structure. The postponement of the desire to become a mother for sociological reasons has led to various reproductive problems in patients of advanced maternal age, and oocyte donation has become the only alternative for these patients due to decreased ovarian reserve and decreased oocyte quality. This has led to a significant increase in oocyte donation around the world, with the particular risk that the genetic makeup may not be preserved, and future generations may not carry the same genetic makeup as the family. The alternative to that would be mitochondrial transfer, which offers the possibility and hope that patients can pass on their DNA structure to future generations.
Conclusion
The results of this study show that pronuclear transfer can result in a high blastocyst formation rate (49.2%), euploidy rate (69.5%) and live birth rate (48.3%). This was true among patients across all age groups, particularly in older patients who often show diminished IVF success rates.
The main disadvantage of pronuclear transfer is that, ethically, the zygotes of elderly patients undergoing nuclear transfer are to be destroyed. Another disadvantage of pronuclear transfer is the higher risk of micromanipulation compared to spindle transfer. Due to the size of the nucleus, the use of a biopsy pipette with a larger diameter ratio may pose a risk. But with a well-optimized protocol, this risk could be eliminated. The main advantage of pronuclear transfer over spindle transfer is that the nuclei are more visible with thermo-microscopy than spindles. The most serious risk of spindle transfer is that the quality of spindle imaging may be poor and that an ICSI procedure, which is an additional micromanipulation procedure, has to be performed after the spindle transfer. This procedure is also more financially costly compared to the pronuclear procedure. In this sense, a more appropriate legal structure needs to be established in nations, and this technique needs to be further developed through further studies. It would be highly beneficial if legal platforms and ethics committees could ensure that this technique can be used clinically as an appropriate method. The fact that the technique poses no risk of micromanipulation, that the resulting children do not carry any risk of mitochondrial mutations, and that the high blastocyst rate indicates that the technique may provide a significant social advantage in the future.






