Manuscript
Introduction
The endometrium is composed of two layers. The functional layer adjacent to the uterine cavity, contains the luminal epithelium, glands, and stroma, and responds to the sequential effects of circulating estradiol (E2) and progesterone (P4) to proliferate and then become secretory during each menstrual cycle, and differentiates to sustain blastocyst implantation. The basal layer contains stem cells which regenerate the functional layer upon menstruation. The functional endometrium consists of a single layer of columnar epithelium and a layer of connective tissue (stroma) containing a rich blood supply provided by the spiral arteries, that varies in thickness according to cyclic sex hormonal influences.
In the endometrium, simple tubular glands reach from the endometrial surface through to the base of the stroma, and upon differentiation provide an optimum environment for implantation and growth of the embryo. The endometrium is central, echogenic (detectable using ultrasound scanning), has a typical trilaminar appearance reaching an average thickness of 7 to 10mm during maximal estrogenic effect, and cohabitates with a unique microbiota. Upon fertilization, the egg may implant into the uterine wall and provides feedback to the ovaries through human chorionic gonadotropin (hCG) secreted by the trophoblast, that rescues and maintains the corpus luteum span, which will continue its role of secreting P4 and E2 and other hormones until the newly formed placenta takes over the endocrine functions.
At a cellular-molecular level, implantation is nowadays recognized as a controlled inflammatory response. After ovulation, a dramatic differentiation process of endometrial stromal cells into decidual stromal cells is induced. Decidualized stromal cells are key players which initiate a cascade of events leading to recruitment and local differentiation of the decidual uterine natural killer cells (uNK cells). These cells mediate embryo recognition and immunotolerance, and secrete cytokines required to activate and control trophoblast invasion. Decidual uNK cells also participate in spiral artery remodeling and activate and control trophoblast invasion(1).
Luteal phase dysfunctions, uncontrolled trophoblast invasion of stroma and arteries, and abnormal decidualization and placentation, can derive in severe pathologies ranging from lack of implantation to miscarriage, development of trophoblast tumors, and can be the culprits of later obstetrical complications such as fetal growth retardation and preeclampsia. Despite active research, particularly derived from the experience and knowledge gained in infertility treatment via in vitro fertilization and embryo transfer (IVF-ET), the critical regulatory elements that determine implantation and regulate its different phases remain incompletely resolved.
Here, it is our primary aim to discuss the state-of-the-art of the orderly sequence of events that characterize the human endometrial cycle, the key molecules and mechanisms involved, their cellular origins and temporal-spatial interactions, and the characterization of the determinant processes of decidualization and the window of implantation (WOI).
The human endometrial cycle: proliferative and secretory phases and establishment of embryo receptivity
The intraovarian processes of follicular recruitment, selection, and dominance, that occur during the proliferative phase allow for an increasing and sustained secretion of E2, which is the main endometrial regulator in the first half of the cycle. Importantly, whereas many ovarian follicles begin their developmental course at each cycle, typically only a single follicle sustains its inherent gametogenic potential; all others succumb to atresia finally having forfeited their latency(2,3). Upon the completion of the follicular phase and ovulation, the dominant follicle undergoes dramatic morphological and functional changes to become the corpus luteum. This robust endocrine organ biosynthesizes and secretes large amounts of P4, the key hormone that prepares the estrogen-primed endometrium for embryo implantation(4). During the mid secretory phase, the endometrium transforms into a temporally receptive tissue that is suitable for embryo adhesion and attachment for a limited period(5,6).
There is a complex and reciprocal paracrine communication between the pre-implanting blastocyst and the endometrium. The pre-implantation embryo signals its presence to the mother by endocrine modulators, such as hCG, and paracrine growth factors, which act locally on the endometrium to facilitate attachment. The intimate mechanisms that determine initial adhesion between trophectoderm cells and the endometrium are not completely understood. In the sheep, it is likely that embryonic L-selectins and their specific ligands present in the luminal epithelium, as well as trophoblastic integrin receptors and the reciprocal epithelial integrin molecules (also including osteopontin and arginylglycylaspartic acid -RGD) play an important recognition role(8). The significance of L-selectins, glycodelin, and other complex sialylated N-glycans and other carbohydrate moieties in human embryo recognition and adhesion has been described(9,10). Following attachment, the embryo penetrates the luminal epithelium, breaches the basal membrane, and invades into the underlying stroma, while in synchrony endometrial stromal cells begin full decidualization(7).
The sequential actions of E2 and P4 after ovulation regulate the formation of a differentiated endometrial stromal tissue, known as the “decidua,” which supports embryo growth and maintains early pregnancy(5,6). Decidualization occurs during the ovulary cycles, independently of the presence of an embryo in the uterine cavity (as opposed to other animal species). Decidualization consists of the differentiation of elongated, fibroblast‐like mesenchymal cells in the endometrial stroma to rounded, epithelioid‐like cells. This morphological change is initiated during the mid‐secretory phase of the menstrual cycle because of elevated P4 levels and begins with stromal cells surrounding the spiral arteries in the upper two‐thirds of the endometrium. Human decidualization begins approximately 6 days after ovulation, at the onset of the putative WOI(11). The process is accompanied by secretory transformation of the uterine glands, an influx of the specialized decidualized uNK cells, and vascular remodeling to support the maternal blood supply to the growing conceptus(12).
Progesterone is an essential regulator of decidualization and a prerequisite for successful blastocyst implantation. But decidualization is also controlled by complex interactions of transcription factors, cytokines, and signaling pathways. A critical network for the decidualization of endometrial stromal cells is comprised of P4 and its downstream molecules, including the transcription factors FOXO1, HOXA10, C/EBPβ and HAND2, and the protein BMP(5). During decidualization, differentiating stromal cells carry a molecular signature of mesenchymal–epithelial transition as they are reprogrammed to become decidualizes stromal cells with widespread changes in gene expression(13). These decidualized cells contribute to the micro‐environment in the human endometrium and have direct and indirect influences on endometrial remodeling, local immune response regulation, antioxidant responses, and angiogenesis(5).
Estradiol-dependent epithelial cells proliferation is a mandatory pre-requisite for adequate decidualization. In these cells, E2 upregulates E2 receptors (ER) as well as P4 receptors (PR). In stromal cells, and after the LH surge, E2 acting via ERα stimulates P4 resulting in proliferation and differentiation of stromal cells. Cyclic AMP (cAMP) of yet unknown but probably stromal origin potentiates the P4 effects. Stromal cells are then included to produce, among others, prolactin and IGFBP-1, that are typically used as biomarkers of decidualization in vitro. Possible epithelial cell signals also participate in stromal decidualization. Importantly, E2 induces angiogenesis by stimulating secretion of VEGF by various cell types (decidualized stroma cells, uNK, and epithelial cells)(14, 15).
Most functions are P4-dependent and occur via genomic progesterone signaling pathways mediated by the nuclear PR, although certain signaling occurs via non genomic PR and other pathways that involve adhesion molecules leading to cell-cell interactions (integrins and others), vasoactive factors (prostaglandins and others), cytokines (such as leukemia inhibitory factor -LIF- and interleukins), homeobox genes, and many other transcriptional factors(13). Further research is needed to determine if these pathways function independently, in parallel, or converge to a common signaling pathway to establish the network of crosstalk between the embryo and endometrium that is necessary for implantation(16).
Prior to implantation, the blastocyst must hatch out of its acellular glycoprotein coat, the zona pellucida. Blastocyst hatching is believed to be regulated by both dynamic cellular components such as actin-based trophectodermal projections, and a variety of autocrine and paracrine molecules originating from the blastocyst and probably also of endometrial origin. Embryonic signals occur via EGF receptor (EGFR) and Cox-2, in coordination with the stimulation of zona pellucida lysins (Heparin sulphate, uPA, Plasmin, MMP-9, and implantation serine proteinase 1 [ISP1]). Pro-inflammatory (IL-6, LIF, GM-CSF) and anti-inflammatory (IL-11, CSF-1) cytokines modulate hatching rates and regulate proteases (MMPs, tPAs, cathepsins and ISP1). There is evidence of endometrial origin for hatching; putative secreted endometrial factors may include Heparin sulphate and EGF, among others(17). Recent IVF data from time-lapse video cinematography demonstrate blastocyst hatching in association with contraction and zona pellucida rupture as captured in vitro(18).
Extensive paracrine relationships exist among the various cell types of the endometrium(14,15,16). The epithelial cells possess ERα, ERβ, and PR, and secrete many functionally significant proteins such as glycodelin A and LIF among others. The stromal cells have ERα, ERβ, and PR in the secretory phase., and secrete a variety of regulatory molecules, including Il-15. Estradiol up regulates ER and PR during the proliferative phase, but down regulates ER during the secretory phase. Differentiated uNKs possess ERβ and receptors for Il-15 (Il-15R), a crucial pathway for immunological regulation. Il-15 stimulates proliferation of uNK cells (pointing to a critical stromal cells-uNK cells interaction). Uterine NKs are cytolytic and cytotoxic, secrete other cytokines (LIF, TNFα, IFNγ, GM-CSF, Il-10), and angiogenic molecules (VEGF and angiopoietin). The epithelial cells lose PR receptors after ovulation, but decidualized cells maintain PR. Estradiol also may exert effects on uNK cells indirectly via cytokines secreted by stromal cells.
Robust angiogenesis takes place during the secretory phase with development of spiral arterioles and a subepithelial capillary plexus. At the time of this extensive neovascularization, endothelial cells exhibit ERβ and have abundant VEGF receptors (VEGFR). VEGF and angiopoietins are the major regulators of endometrial vessel formation, maintenance, stabilization, and regression. VEGF and its receptors (VEGFR) play a significant role in endometrial angiogenesis and participate in the regulation of other endometrial functions. VEGF mRNA and protein are present in glands and stroma; VEGF protein can be identified in neutrophils; and VEGF mRNA is present in uNK cells. VEGFR-1 and -2 are present on endothelial cells and stroma. VEGFR-3 is present on lymphatic cells(15).
Endometrial immunology has been extensively characterized. Immune cells include: uNK, macrophages, and other leukocytes. The uNKs are phenotypically unique (CD56b₊, CD16- and CD3₊ (as opposed to peripheral or systemic NK cells that are CD56d₊, CD16b₊ and CD3-). Their origin is still controversial, as it is unclear whether they are derived from in situ proliferation versus de novo recruitment and migration from leukocyte subtypes from blood. On the other hand, uterine macrophages act as oxygen sensors and secrete VEGF and angiopoietin. Neutrophils populate the endometrium before menstruation, and T cell lymphocytes constitute 45% of immune cells(14,15,16).
In normal pregnancy, the trophoblast invades the maternal endothelium releasing microvesicles and soluble mediators (such as TNFα) into the maternal circulation, leading to a low-level physiological inflammatory response that is a characteristic feature of trophoblast adhesion and invasion(1). TNFα also induces expression of other cytokines, such as IL-6 and IL-8, with modulatory functions in angiogenesis, neutrophil migration, and differentiation. In support of this concept, it has been shown that abnormal inflammation due to elevated levels of TNFα is associated with miscarriages and adversely affects the viability and implantation competence of preimplantation embryos(19).
Endometrial secretions in the uterine cavity contain mediators important for endometrial receptivity and embryo implantation. Extracellular vesicles (exosomes and microvesicles) have been characterized in embryo-endometrium crosstalk(20, 21). Proteomic studies of the human endometrium and uterine fluid (secretome) suggested a pathway to biomarker discovery(20,21).
After ovulation, endometrial stromal cells and perhaps mesenchymal stem cells can be transformed into decidua stromal cells. Cytokines secreted by decidua stromal cells and by recruited leukocytes into the functional layer maintain a pro-inflammatory environment(1). After the embryo implants, decidual stromal cells generate a ‘wave’ of decidualization by autocrine and paracrine cytokines that spread throughout the uterus(23). Decidual stromal cells significantly induce uNK cells proliferation and differentiation by secreting IL-15(24). Multiple cytokines and angiogenic factors secreted by decidua stroma cells, uNK cells, and macrophage cells induce uterine spiral arteries to remodel. Meanwhile, uNK cells and decidual stromal cells can control extra villous trophoblast cells invasion and “sense” embryo quality (see below)(25). Single-cell sequencing showed that uNK cells in early pregnancy are divided into various subpopulations that differ in surface receptor expression profiles and cytokines secretion, with unique functions including cells destined to combat microbial infections, to determine immune tolerance, remodel spiral arteries. and stimulate fetal growth. It is speculated that functional alterations of one or more of the human uNK cells subpopulations may result in pregnancy complications such as miscarriage and preeclampsia(26).
In the absence of pregnancy, the endometrium enters the menstruation phase because of progesterone withdrawal. Progesterone withdrawal initially affects cells with PR resulting in extensive vasoconstriction and cytokine changes. Chemokine release and chemotaxis determine invasion and activation of neutrophils, with a cascade of events resulting in release of MMPs and tissue destruction. Vascular changes accentuate with hypoxia and secretion of VEGF is augmented. This cascade leads to activation of pro-MMP (MMP-1 and –7, and Il-1) and accentuation of hypoxia. Nevertheless, there is no certainty as to the origin of the MMPs and/or invading neutrophils(14).
As mentioned earlier, it has been postulated that stem cells present in the endometrial basalis may be at least partly responsible for initiating the regeneration process after menstruation. In a pioneer study, clonogenic cells or colony-forming units (CFUs) were identified in purified populations of human endometrial epithelial and stromal cells isolated from hysterectomy tissue(27). These are stem cells located in the endometrial basalis, they represent <1% of cells, and are clonogenic cells, in both epithelial and stromal lineages. Growth is E2-dependent probably through EGF, TGF (transforming growth factor) and PDGF (platelet derived growth factor). These cells differentiate and transit into the endometrial functionalis 27). Novel “omics” approaches have now been characterized to identify and purify the endometrial mesenchymal stem/stromal cell (eMSC) population.
There is proof that migration of bone marrow mesenchymal stem cells bmMSCs to the human endometrium contributes to the endometrial stem cell pool and thereby endometrial renewal(13,28). The change in “niche effect” (different local tissue environments in the endometrium layers) and the differentiation process towards endometrial fibroblasts will alter the migration properties and the cytokine secretion profile of these cells. The bmMSCs, the eMSCs possess high migration activity; during their differentiation process towards stromal fibroblasts there is loss of stem cell surface markers, decreased migration activity, and a subtler cytokine profile likely contributing to normal endometrial function. Progesterone and E2 withdrawal drive endometrial collapse and subsequent hypoxia during the late secretory phase of the menstrual cycle most likely triggering the homing signal for the bmMSCs for the subsequent cycle(28).
Importantly, new investigations are unveiling the potential cell-based therapeutic role(s) of bone marrow-derived and endogenous stem/progenitor cells in endometrial proliferative disorders, including endometriosis, adenomyosis, thin dysfunctional endometrium, and Asherman's syndrome.
Models for the study of human implantation.
Ethical concerns have limited the use of in vivo approaches to study human embryo implantation. Since human implantation sites are not available for experimentation, and animal models may or not represent human physiology, researchers have implemented in vitro culture systems with whole endometrial tissue, primary epithelial and stromal cells, and human established cell lines, to gain insight into human implantation(29).
While fixed human tissue enables identification of the in vivo cellular location of molecules, this approach cannot provide functional data. On the other hand, because of the very limited availability of fresh primary tissue, cell lines provide the tools for most functional studies. But these are far from perfect, and information gained with these models can be subsequently validated in primary tissue or animal models.
In vitro culture models of endometrium have been established from two-dimensional (2D) cell-based to three-dimensional (3D) extracellular matrix (ECM)/tissue-based culture systems(30). Numerous human established cell lines have been used for examination of implantation. They include “receptive” endometrium (luminal epithelium): ECC1, Ishikawa, HES cells; “non-receptive” endometrium (luminal epithelium): HEC-1A cells; glandular epithelium: Ishikawa, RL 95-2; syncytiotrophoblast: BeWo; trophoblast adhesion and migration: AC 19-88, HTR-8/SVneo; trophoblast invasion: JEG 3, Jar, HTR-8/SVneo, BeWo cells; and stromal cells: T-HESC (immortalized)(29).
Wang et al(31) developed a novel model of human implantation consisting of a 3D endometrium-like culture system with fibrin-agarose as matrix scaffold, to study attachment and invasion of human trophoblast cells (Jar spheroids). The model uses either primary epithelial and stromal cells obtained from endometrial biopsies, or established cell lines (i.e., Ishikawa and HESC). Time-dependent experiments demonstrated a high rate of attachment of Jar spheroids to the epithelium, and adhesion was strongly related to the various cell types present in the 3D culture. An architecturally and functionally competent 3D endometrial culture system was therefore established, that coupled with Jar spheroids mimicking trophoblast cells, provides a promising in vitro model for the study of certain aspects of human implantation(30).
Follow up studies(32) demonstrated that the attachment rate of Jar spheroids to the 3D was significantly increased by E2 plus MPA treatment. Analyses of Z-stack confocal and stained optic microscopic images showed that Jar spheroids breached the epithelial cell monolayer, invaded, and were embedded into the 3D matrix in response to decidualization signals. Further heterologous experiments, using mouse blastocysts as surrogates for human embryos, revealed a high degree of attachment (day 1 of co-culture) and embryonic cells breaking of epithelial layer and invasion of stroma (days 2 and 5) (Figure 1).
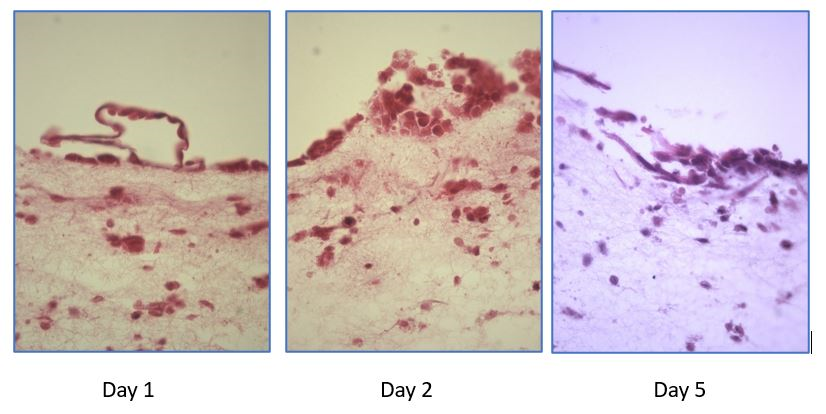
Figure 1. A novel heterologous model of human implantation: 3D endometrium-like culture system to study attachment and initial invasion of mouse blastocysts (Wang H, Oehninger S, and Horcajadas JA, unpublished observations). A 3D culture system was established with Ishikawa and HESC cells in agarose/fibrin/tranexamic acid/CaCl2 scaffold cultured in inserts as detailed in Wang et al(31,32). Mouse blastocysts were grown from commercially available cryopreserved 2-cell murine embryos. Ten expanded blastocysts were seeded on top of the 3D construct per experiment. Day 1 of co-culture: attachment of a hatched blastocyst to the epithelial layer of the 3D construct. Days 2 and 5: initial invasion of the stroma by the trophoblast with breakage of epithelial layer (HE x400).
For long term 3D cultures systems, organoids have been generated from established human adult stem-cells. These organoids expand long-term, are genetically stable and differentiate following treatment with reproductive hormones. Transcript analysis confirmed great similarity between organoids and the primary tissue of origin, representing a novel system to recapitulate early pregnancy events(33).
However, all models described so far have their pros and cons, and there is no single ideal model to study the whole implantation process. Additional studies are needed to establish a comprehensive in vitro model that can recapitulate the biology of trophoblast-endometrium interaction during early pregnancy.
Characterization of the WOI: from histology, through individual molecular markers, to genomics and proteomics, to the identification of the transcriptomic signatures of the window of implantation
The changes in the histologic appearance of the endometrium during the ovarian-menstrual cycle have been well characterized(34). Measurement of mid luteal phase (day 21-22) serum P4 levels and a timed endometrial biopsy have been long used to confirm ovulatory status. However, the value of an endometrial biopsy to ascertain fertile status versus infertility using standard criteria defining an out-of-phase biopsy as a greater than 2-day delay in the histological maturation of the endometrium, has been challenged, leading to its abandonment. It has now been concluded that the histological dating of the endometrium does not discriminate between women of fertile and infertile couples and should not be used in the routine evaluation of infertility(35).
Since the early days of assisted reproductive technologies (ART), a key question has been whether the timing of nidation is dependent on the stage of embryonic development, endometrial maturation, or a possible dialogue between the two (synchrony). Based on an early IVF study of fresh embryo transfers of identical gestational age, it was concluded that the first embryonic signal detection (presumed window of implantation) extends between cycle days 20 and 24(36). In subsequent studies, investigators examined women with ovarian failure, and induced histologically normal endometrial function during a preparatory cycle consisting of sequential administration of E2 and P4. During a subsequent cycle, endometrial stimulation was synchronized with surrogate-embryo transfers and pregnancies were achieved(37).
Moreover, taking advantage of the establishment of donor egg programs, elegant studies aimed to decipher the “window of transfer” in the human(38). In these clinical studies, embryos were transferred on different days of the luteal phase. Embryos were transferred into a defined endometrial bed, characterized histologically as day 17 to 19 endometrium by the criteria of Noyes et al(34). Results strongly suggested that 1- the window of transfer in the human for the 4- to 16-cell embryo extends to day 19 (perhaps day 20) of the idealized 28-day cycle, with the proximal width of the window yet undefined; and 2- that the WOI in the human does not extend beyond day 22 or 23 of the menstrual cycle.
The abandonment of the endometrial biopsy/histologic data as a diagnostic test led to an absence of any reliable diagnostic test to determine the endometrial status. Consequently, the standard workup for infertility in clinics worldwide no longer included endometrial status, beyond a limited use of ultrasound imaging to determine endometrial thickness and pattern. In a secondary approach, numerous authors reported on semiquantitative changes of a defined endometrial molecule known to participate in implantation, comparing its expression in the non-receptive and receptive days of the cycle. Included in this list are LIF, integrins, interleukins, CSF, glycodelin, MUC 1, and others, typically examined by immunohistochemistry(39). Scanning microscopy analyzing presence of endometrial epithelial pinopds was also investigated(40). But it was later agreed that no final conclusions could be drawn about the clinical value of these measurements in the assessment of endometrial function and prognosis for pregnancy after ART(39).
With the advent of the “omics” revolution, endometrial biologywas thoroughly re-examined/. Independent investigators simultaneously reported on wide genomic analysis of human endometrial receptivity (genomics) using high density microarrays and bioinformatics technology. Several gene candidates were identified that could segregate the secretory phase of the human endometrium in natural and ovarian stimulation cycles, as well as changes across the menstrual cycle (41,42,43).
The endometrial receptivity assay (ERA) was the first transcriptomic test developed to diagnose the endometrial receptivity status of infertile patients (44). To identify genes involved in the human endometrial receptivity signature, the authors initially analyzed differences in genome-wide expression profiles between receptive and pre-receptive endometrium using raw expression data from three different models of endometrial receptivity: the natural cycle as the optimal model, the ovarian stimulation cycle as suboptimal, and the refractory endometrium induced by the insertion of an IUD as a negative control (44,45). The ERA was devised as a customized array containing 238 differentially expressed genes that were coupled to a computational predictor able to identify the transcriptomic profiles of proliferative (PRO), pre-receptive (PRE), receptive (R) or post-receptive (POST) endometrial samples, regardless of their histological appearance.
Additional studies showed high specificity and sensitivity for endometrial dating, and the transcriptomic signature was further defined by 134 genes. Clinical algorithms were introduced for embryo transfer personalization during IVF cycles, derived from data suggestive of “displacement” of the WOI in cases of failed implantation and others (45). Over a decade of ERA clinical application there is still controversy as to the clinical significance of the test, populations to be applied to, and others (46,47,48,49). Notwithstanding these caveats, the ERA has highlighted the significance of the transcriptomic endometrial receptivity status, and significantly deepened our basic knowledge. Other variations of this test have lately been introduced in the clinical scenario. As technology evolved, microarray and PCR-based clinical tests were replaced by next generation sequencing technology (NGS). Furthermore, it has been suggested that important clinical information may be obtained by combined analysis of the transcriptomic profiling (ERA-NGS) and uterine microbiota analysis by NGS. Using 16S rRNA gene sequencing it has been reported that the endometrial microbiota composition before embryo transfer is a useful biomarker to predict reproductive outcome in ART (50).
It has been discussed that endometrial receptivity is not an ‘all or none phenomenon’, nor does the analogy of a window indicate that the window opens at a certain point and then closes to any interaction with the embryo(51). In 2020, Wang et al published a very elegant novel study aiming to characterize the human endometrial transcriptome at a single-cell level, revealing cell-specific expression signatures across the menstrual cycle(52). The investigators applied single-cell RNA sequencing (scRNA-seq) to generate an RNA-seq library, followed by gene ontology functional enrichment. The authors were able to characterize the human endometrium across the menstrual cycle from both a static and a dynamic perspective with additional information being provided by constructing single-cell-resolution trajectories of the menstrual cycle.
Employing canonical markers and highly differentially expressed genes, Wang et al identified six endometrial cell types: epithelial and endothelial cells, stromal fibroblasts, macrophages, lymphocytes, and a novel ciliated epithelial cell type(52). The ciliated epithelium is a distinct endometrial cell type with its own signature; these cells are consistently present in the healthy endometrium but dynamically changing in abundance across the menstrual cycle. Importantly, information was provided for the first time on gene expression modifications occurring at the estimated opening and closure of the WOI. Based on their data, the human WOI opens with an abrupt and discontinuous transcriptomic activation in unciliated epithelia, accompanied by a widespread decidualization feature in the stromal fibroblasts(52,53).
The term “endometrial receptivity” implies a passivity of function in implantation that recent discoveries have come to challenge(13, 54). Additional functions have been ascribed to the decidualized stromal compartment of the endometrium indicating that the decidua has a key role in directing the maternal response to the implanting embryo. It is speculated that migration of the decidualized stromal cells is controlled by transcription factors, chaperones, cytokines and trophoblast factors, and results in a regulatory system, which requires balancing of endometrial and embryonic phenotypes to modulate implantation(54,55).
The need for such “biosensor function” becomes clear when one considers the challenge that the implanting embryo presents to the endometrium. In contrast to other species, human embryos are characterized by their high rate of chromosomal abnormalities. Most aneuploidies will fail to establish an ongoing pregnancy, despite being invasive enough to initiate implantation. Although this may in part reflect incompetency, it has become evident that there is also an active maternal strategy to prevent investment in these invasive but poorly viable embryos. Aneuploidy is associated with proteotoxic stress, metabolic overdrive, and production of proteases, embryonic conditions that can be “sensed” by the decidua. If decidualization is suboptimal, then the biosensor function may be disrupted too. The consequence of this could be that rather than allowing early rejection of poor-quality embryos before the mother becomes aware that she may have conceived, the endometrium would allow poorly viable embryos to establish a clinical pregnancy, ultimately destined to fail, and present as a clinical miscarriage. Persistently impaired endometrial selectivity would result in recurrent early pregnancy loss. Conversely, an excessive decidual response would allow receptivity to dominate, reducing the incidence of miscarriage but increasing the likelihood of implantation delay or implantation failure after IVF(13,55,56,57).
At the current stage of knowledge, it appears that both concepts of receptivity (i.e., effective and timely opening and closure of the WOI under normal conditions, and pathological displacement of the WOI in groups of sub fertile women that can be corrected by modifying embryo transfer timing), and selectivity (power of decidua to accept/reject good/poor quality embryos, or function as sensor/driver of pregnancy health), may coexist.
Conclusions
We are witnessing an era of precise medicine, perhaps to be augmented by utilization of artificial intelligence, with incorporation of personalized medicine, versus a controversial philosophy that “one size (shoe) fits all”. The data presented herein unequivocally highlights the complexity of the endometrium, the numerous cascades of control, and the fact that sophisticated mechanisms may coexist and indeed may be complementary to each other to determine embryo implantation.
From a basic physiologic point of view, we have highlighted that endometrial receptivity has been defined at cellular and transcriptomic levels. Novel data confirm that the WOI clinically extends between days 20-24, and transcriptionally lasts 30–36 hours and, depending on the patient, occurs between LH + 6 to LH + 9 in natural cycles or from P + 4 to P + 7 in hormonal replacement therapy cycles(53). The gene expression changes, and temporal patterns associated with opening and closure of the window, particularly at the level of the unciliated epithelial cells, are starting to be understood. Furthermore, the stromal decidual cells and uNK play a critical complementary regulatory role of embryo selection acting as biosensors that drive the normalcy of the implantation process and protect the health and growth of the embryo (Figure 2).

Figure 2. Endometrial receptivity and selectivity: characterization of the WOI. Diagram showing a panoramic view of endocrine events during the normal ovarian-menstual cycle, centered on the day of the LH surge, and the temporal transcriptome dynamics of endometrial transformation across the WOI obtained by single-cell RNA sequencing. Concomitant trasncriptomics changes during the WOI and the functional processes occurrung at the level of 1-epithelium (embryonic recognition and attachment, initial blastocyst stromal invasion); and 2-Stromal cells (decidualization with differentiation of sromal cells and uNKs, immunomodulation, neovascularization, embyonic controlled invasion) are depicted. Current evidence indicates that the WOI lasts 30–36 hours and, depending on the patient, occurs between LH + 6 to LH + 9 in natural cycles or from P + 4 to P + 7 in hormonal replacement therapy cycles(52,53) Note different timing and patterns of cellular and gene activation at opening and closure of the WOI. Receptivity(45) and selectivity(56) may represent two complemetaty mechanisms that regulate implantation of a healthy embryo. B: blastocyst. ES: early secretory; MS: mid secretory; LS: late secretory phase.
From the point of view of novel technologies, the future appears to be bright. High throughput genomics methods allowed in-depth study of early implantation and development. RNA sequencing (transcriptomics) represents an average of gene expression patterns across thousands to millions of cells. On the other hand, scRNA-seq reveals RNA abundance. Single-cell RNA sequencing involves isolation of single cells, capturing their transcripts, and generating sequencing libraries in which the transcripts are mapped to individual cells at unprecedented resolution. However, it captures only static snapshots at a point in time. This information can now be extended by calculation of RNA velocity -the time derivative for dynamic biological systems such as endometrial differentiation and implantation, and embryo development, that are based on processes of cell differentiation and fate, transitions, and lineage(58).
RNA velocity, the time derivative of the gene expression state, can be directly estimated by distinguishing between unspliced and spliced mRNAs in common single-cell RNA sequencing protocols. Single-cell RNA sequencing successfully captures the heterogeneity that results from processes such as development, reprogramming, and regeneration, but it loses lineage relationships, since each cell can be measured only once. To mitigate this problem, scRNA-seq can be combined with lineage tracing methods or metabolic labeling methods that use the ratio of nascent to mature RNA molecules to link observed gene expression profiles over short time windows. Yet both strategies are mostly limited to in vitro applications, prompting the development of computational approaches to reconstruct pseudotime trajectories(59).
In addition to transcriptomics, investigators have now been able to measure protein translation with spatially resolved, single-cell resolution. Recently, Zeng et al.(60) developed a highly multiplexed, ribosome-bound messenger RNA imaging technique called RIBOmap and applied it in single cells in situ to profile translation events with spatial coordinates. The pairwise spatial translatomic and transcriptomic mapping enabled the authors to systematically identify cell type- and tissue-region–specific translational regulation, paving the way for uncovering novel posttranscriptional gene regulation principles and mechanisms that shape the proteome for cellular and tissue functions. In the end, physiological processes and diseases need to be understood in terms of proteomics and metabolomics, which define individual phenotypes and functions in systems biology.
But unfortunately, this fast pace of discovery has not yet been matched by new clinical applications in infertility and ART, and much more work is needed to decipher endometrial pathologies that result in implantation failure and other challenging diseases. There is still no agreed test to study the WOI(47,48,51) and no novel management options have been introduced. But we remain enthusiastic that the new basic information, with surely more data yet to arrive, will lead to improved diagnostic tools and therapies for implantation and other gynecological disorders, and increased ART efficiency.



