Manuscript
Introduction
Embryo implantation and a correct pregnancy development is the result of a combination of different factors, including an optimal anatomical structure of the uterus, and adequate hormonal and molecular signaling.
Firstly, for correct implantation to occur there must be good synchrony between the endometrium and blastocyst. The blastocyst should be competent for implantation, and the endometrium must be receptive, that is, it needs to be sufficiently mature for the trophectoderm to adhere to the endometrial epithelial cells and invade the endometrial stroma and vasculature. Among the factors that contribute to altering receptivity, inflammatory events seem to play a key role in the process(1).
Nevertheless, endometrial receptivity (ER) is not the only crucial factor for correct embryo implantation, elements affecting endometrial functionality also play a critical role. Endometrial functionality refers to physiological functions of the uterus, which include menstruation, preparation for implantation and maintenance of pregnancy if implantation occurs(2). In this context, the immunological homeostasis of the uterus and the state of the local microbiome actively participate in the establishment of this correct physiological competence.
The endometrial mucosa has the peculiarity of being able to generate an immune response to infections and, in turn, tolerate external agents such as sperm and embryos. The superficial endometrium contains immunological cells that gradually mature during the menstrual cycle. These cells have a peripheral origin and respond to the expression of chemokines and cytokines regulated by sex hormones. An alteration in the composition of this cellular profile at the intrauterine level, such as the appearance of an inflammatory profile, can affect the endometrial function. In this context, it is known that inflammation can be triggered by an altered endometrial microbiome(3,4).
Advances in molecular biology make it possible to analyze the microbiome with high levels of identification and to design methods for its monitoring. Culture-based microbiological techniques are subjective and not sensitive enough, nor adequate in some cases, to identify microbiomes because some bacteria have no capacity to grow in culture. For instance, real-time quantitative Polymerase Chain Reaction provides a rapid and an efficient detection of target genes that allow microorganisms to be identified at the strain level, compensating for limitations of other techniques such as the time of operation and costs of Next Generation Sequencing (NGS) technology. Furthermore, NGS, which is based on the sequencing of the 16S rRNA gene, produces problems in identification at the species level, caused by high sequence similarity within this gene, and omits the detection of other non-bacterial microorganisms(5). These techniques have made it possible to demonstrate that the uterus is not a sterile niche, as was traditionally believed. Both anaerobic and facultative aerobic microorganisms are found in the female tract, with a bacterial community dominated by Lactobacillus genus(6).
Furthermore, it has been possible to demonstrate that the uterine microbiome also appears to be influenced, under certain conditions, by other local microbiomes (figure 1). Dissemination through the bloodstream of microorganisms originating from other mucosal locations occurs due to ruptures of the epithelial barrier. In addition, there are several factors that promote the colonization of vaginal microorganisms towards the uterus, such as assisted reproductive technology (ART) procedures, the semen colonization itself, or the introduction of intrauterine devices, among others(3,7,8).
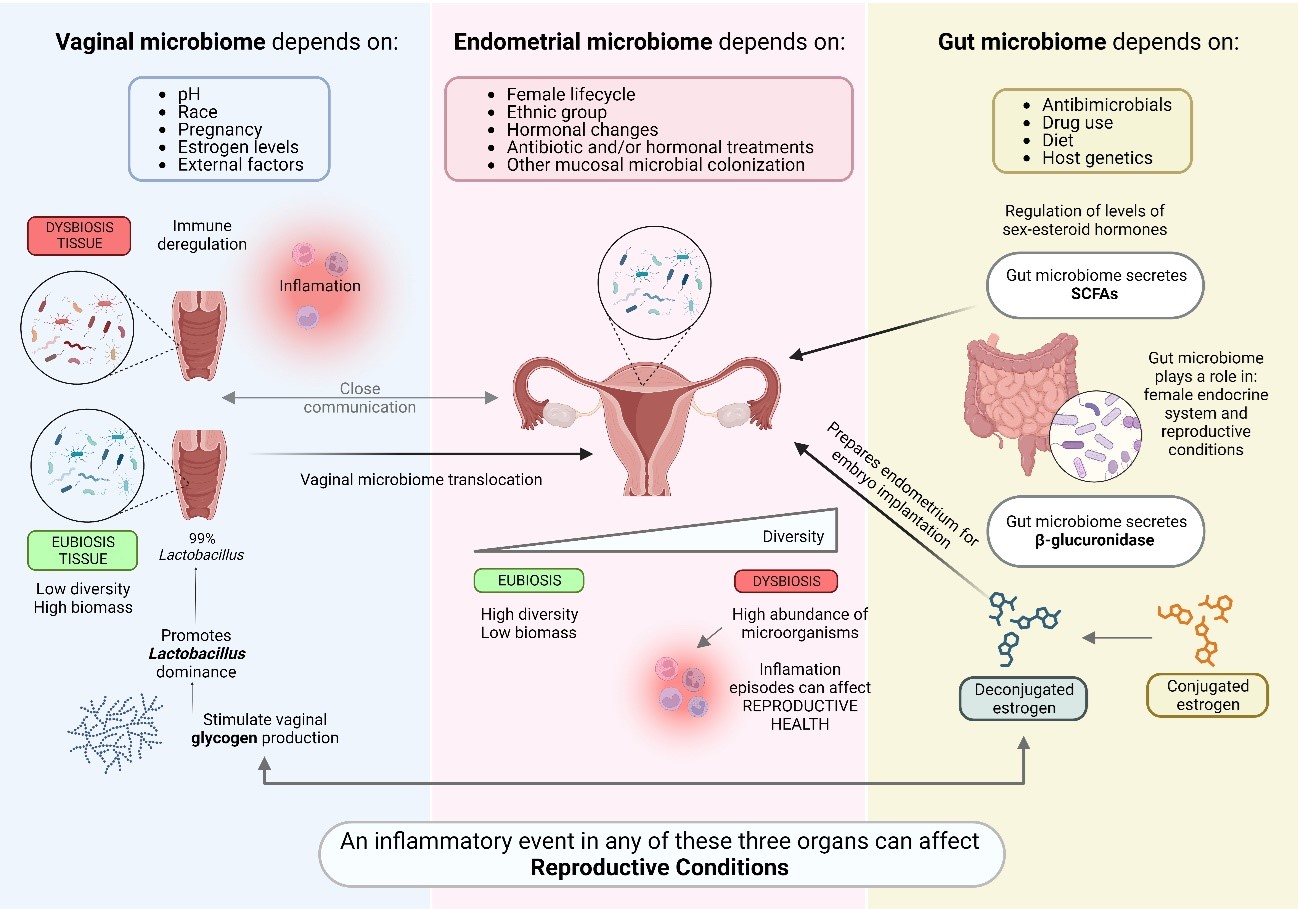
Figure 1. Interconnection between the endometrial microbiome and other mucosal tissues.
This review aims to describe the current state of art of the endometrial microbiome and its influence on endometrial functionality. In addition, the impact of other local microbiomes, such as the vaginal and intestinal ones, on the endometrial microbiome and in turn on its functionality will be addressed.
Methods
The bibliographic searches were conducted with a specific focus on the past decade (January 2013 to September 2023). These searches employed a carefully chosen set of keywords, such as "endometrium”, “functionality”, “immunology”, "receptivity", “vaginal”, "gut", "microbiome”, “probiotics”, “implantation”, among others, to ensure alignment with the review's objectives. These keywords were combined using Boolean operators (AND, OR, and parentheses) primarily within several databases: Pubmed, SCOPUS, ScienceDirect and the online web of World Health Organization.
To be considered for inclusion in the review, the selected bibliography needed to meet specific criteria:
It had to encompass reviews, observational studies, and randomized clinical trials.
The publication date needed to be within the past decade.
The documents were required to feature titles and/or abstracts containing information pertinent to the study objective.
Furthermore, exclusion criteria were applied to exclude articles that did not align with the scope of the review:
After an exhaustive screening process, a total of 40 articles that fully satisfied the eligibility criteria were identified.
Impact of the microbiome in the endometrial function
Endometrial microbiome
The endometrium is the outermost layer of the uterus, and its main function is providing optimal conditions for a correct embryo implantation. This organ has traditionally been considered a sterile niche. However, thanks to the Human Microbiome Project and the development of precise and effective molecular biology techniques, this hypothesis changed; approximately 9% of the total human microbiome was found in the female reproductive system(9).
One of the main factors for embryo implantation is that there is a balance in the microbial composition of this tissue. Microbiota is defined as a group of living microorganisms within a particular environment, and the microbiome as whole, these microorganisms and their “theatre of activity”. The latter involves the whole spectrum of molecules produced by the microorganisms, including their structural elements, metabolites, and molecules produced by coexisting hosts and structured by the surrounding environmental conditions(10). Groups of microorganisms that colonize the endometrium include 85% bacteria, 10% fungi, 5% viruses and 0.3% archaea(11).
Numerous studies describe that woman uterine microbiota presents a low alpha diversity with a high abundance of species of the genus Lactobacillus such us L. crispatus, L. iners, L. jensenii and L. gasseri. These species are mainly responsible for maintaining optimal conditions in the tissues to increase the chances of embryo implantation, thanks to their properties that reduce pH and protect against the invasion of pathogens and the levels of pro-inflammatory parameters(9,12,13). At the level of endometrial immunology, the production of lactic acid from Lactobacillus limits the toxicity of Natural Killer (NK) cells, promotes blood vessel regeneration processes and modulates the immune inflammatory response mediated by cytokines and other immune cells(14,15).
In addition to lactobacilli, other bacterial genera have been identified in endometrium thanks to metatranscriptomic analysis such us Bifidobacterium, Corynebacterium, Gardnerella, Prevotella or Staphylococcus, among others(16). Unlike in other areas of the body, a high variety of microorganisms in the endometrium can result in a dysbiotic state and the subsequent development of some pathologies(16).
It is considered that endometrial tissue is in eubiosis state when the percentage of bacteria of the genus Lactobacillus is equal or greater than 90% of the total microorganisms abundance, being this condition responsible of maintaining a balanced tissue(9). It is important to consider microbial typification when identifying endometrial microbiota. In general terms, obtaining a genomic index of the species L. crispatus and L. gasseri within its range, and a Lactobacillus percentage greater than 90% as a result, reports an optimal endometrial microbiota status; however, sometimes this percentage may also be due to the dominance of species such as L. iners, whose capacity to produce beneficial substances is lower, being harmful and making tissue conditions not optimal for embryo implantation to occur(17). Therefore, even though most research on reproductive microbiology determines endometrial status based on a percentage of Lactobacillus greater or less than 90%, the molecular technique applied need to be considered and the relationship between the concepts of eubiosis and Lactobacillus dominance should be re-examined(9).
Despite the predominance of bacteria of the genus Lactobacillus in the endometrial microbiota of most women, composition and individual microbial profile is highly dynamic and can change over time causing episodes of imbalance or dysbiosis. This balance can be altered by different causes including female lifecycle, ethnic group, hormonal changes, some pathologies, antibiotic treatment, and the use of intrauterine devices, among others(18). During the menstrual cycle, natural hormonal fluctuations can have consequences on the microbial composition of endometrial tissue. For instance, the percentage of Lactobacillus increases during follicular development, reaching its peak in the luteal phase; and after menstruation, the proportion of these bacteria is decreased. Other species belonging to the Prevotella and Sneathia genus increase during the proliferative and secretory phase(19).
On the other hand, different studies have shown that women subjected to ART with exogenous hormones administration, suffer modifications in their endometrial microbiota. In particular, bacterial diversity boost while the proportion of bacteria of the genus Atopobium and Prevotella increase and the percentage of Lactobacillus decreases(20).
All these factors, can cause negative effects in endometrial functionality, producing inflammation episodes that can affect reproductive health, leading in turn to intra-amniotic infections, premature births, spontaneous miscarriages, and infertility through mechanisms such as alterations of vascular and immune cell functions(12). Additionally, imbalance in the microbiome can trigger diseases such as chronic endometritis, which is most commonly caused by chronic bacterial infection at the uterine level(8).
From a clinical point of view, efforts are focused on the search for specific, complete and individualized treatments capable of improving pathologies caused by microbial imbalances, usually based on antibiotics, especially to treat infections such as bacterial vaginosis or prevent premature birth. However, the administration of these drugs to improve microbial balance before embryo transfer is controversial. The lack of specificity of broad-spectrum antibiotics could impair not only the growth of dysbiosis-causing microorganism, but also protective lactobacilli.
A possible strategy to modulate the reproductive microbiota is the combined use of antibiotics/antifungals with prebiotics and probiotics, which include, among others, live microorganisms of the genus Lactobacillus(21). The use of these compounds could offer an interesting approach to restore the microbiota while avoiding the disadvantages of antimicrobials, such as resistance to them, the high rate of recurrent infections after treatment, and the side effects that could appear derived from the elimination of microbiota from other parts of the body. In addition, microbiota transplants are also gaining popularity to improve and maintain the optimal composition of the microbiota in order to benefit human health(22).
The role of the endometrial microbiota in the embryonic-maternal relationship during the beginning of pregnancy establishment is generating great interest in the field of reproductive medicine. A better understanding is needed of what optimal endometrial tissue means, how to achieve it, and what factors would improve the reproductive success of subfertile women with reproductive desires.
Vaginal microbiome
At vaginal level Lactobacillus is also the most dominant member of the local community in most healthy women of reproductive age. The most common species present vaginally include L. crispatus, L. iners, L. gasseri, and L. jensenii, with a community states (CST) described depending on the composition of these species to classify the vaginal microbiome(12).
The vaginal microbiota is also very dynamic during woman life and highly dependent on estrogen levels. The stability of this local microbial community depends on many other internal factors such as race, pH, pregnancy or menstruation, but also on external factors(6,12,23). Furthermore, a good immune regulation is also necessary for vaginal microbiome maintenance. Disturbance of this immunological balance can lead to an acute inflammatory reaction or an insufficient immune response. In a normal state, commensal communities maintain a barrier and a stable mucosal environment and a correct interaction with the immune system through their metabolites. These mechanisms result in the activation of uNK cells and the regulated development of specific T cell subsets, essential steps for immunotolerance of the fetus(4).
The microbiomes of the upper and lower sections of the reproductive tract are unique and specific. The total biomass is much lower at the endometrial level compared to the vagina. At the vaginal level, the lower pH makes the conditions unfavorable for most microorganisms and therefore there is less diversity compared to the endometrium. These differences become smaller with circumstances such as aging, vaginal births and miscarriages. Furthermore, the existence of vaginal microbiome translocation to the endometrium due to different mechanisms and circumstances such as uterine contractions and instrumental manipulation is well known(6,8,12,24,25). In fact, vaginal location is the most common source from which uterine colonization occurs, but the physiological significance of this translocation is still unclear(8,26).
It is important to note that, as well as at the endometrial level, at the vaginal location there are not exclusively bacterial microorganisms either. For instance, in the female reproductive tract vulvovaginal candidiasis accounts for 20-25% of all vaginitis. Several risk factors are known that can lead to the colonization of Candida into the uterine cavity, which may have an impact on a pregnancy, including the use of intrauterine devices, embryo transfers or underlying medical conditions(7,27).
On the other hand, a variety of vaginal DNA viruses have been also identified in generally healthy and asymptomatic women of reproductive age, such as the Adenoviridae, Herpesviridae or Papillomaviridae families. It is not yet clear if there is a core vaginal virome, since the function of this virome at the vaginal level is still unknown. What is likely is that viruses can influence the immunological profile present in the mucosa, and that their presence seems to be influenced by Lactobacillus(3,12,24). In addition, the presence of phages has also been detected at the vaginal level. It is believed that they can influence the configuration of the local microbiota, protect pathogens from immune system and affect the health of the host to a certain extent(3).
All this complex and dynamic vaginal microbial community is highly important for reproductive outcomes, specially at the first stages of pregnancy. It plays a key role in preterm labor and spontaneous birth, being a stable microbiome, including viruses and fungi composition, more related to spontaneous births(3,12,28).
Vaginal microbiota in pregnant women with spontaneous births is reported to be less rich and less diverse compared to the non-pregnant vaginal microbiota(12). Vaginal dysbiosis state, which is characterized by a non-Lactobacillus genus dominance, is linked to adverse outcomes such as premature birth and sexually transmitted diseases(6,12,23).The main contributing factors for these adverse clinical results include intra-amniotic infections, ascending infections, cervical insufficiency, stress, vascular disorders, etc. Ascending genital infections seems to alter the delicate maternal-fetal immune balance by releasing toxins and a series of enzymes that compromise the fetal membranes(12).
In addition, the predominance of anaerobic bacteria appears to have a negative impact on the outcome of ART. Patients who do not become pregnant have a higher abundance of pathogens such as Gardnerella, Enterococcus, Streptococcus, Staphylococcus and Prevotella, among others, while pregnant patients have a higher abundance of Lactobacillus(26,29,30). Furthermore, a balanced vaginal microbiota seems to also modulate important metabolites for embryo implantation, such as glycerophospholipids(30). However, there are studies which do not find significant differences between pregnant and not pregnant woman at vaginal microbiome level(25) probably due to the difficulty in microbiome profile characterization analyses and the fact that most studies focus on exclusively analyzing the bacteriome.
Ultimately, the close communication of the vagina with the endometrium and its influence on endometrial health make the vaginal microbiome the first essential barrier for a correct composition of the microbiome of the general reproductive tract, being key factor for the establishment and maintenance of pregnancy.
Gut microbiome
The gut microbiome encompasses the complex network of bacteria, viruses, fungi, archaea and protozoa residing within the gastrointestinal tract, as well as their genome and metabolites. This microbiome is recognized as an endocrine organ indeed, wielding the ability to exert influence over the intestinal environment and impacting distant organs, as well as several interconnected biological pathways. The preservation of a robust and balanced microbiota is essential for host well-being, since microbial community actively participates in digestive process, promotion of immune cell maturation, and detoxification(31).
However, the composition and dynamics of the gut microbiome are subject to a multitude of factors, which include dietary choices, host genetics, among others(31).
The gut microbiome plays a pivotal role in regulating the female reproductive endocrine system and significantly impacts female reproductive health and associated conditions. The human microbiome exerts its influence across all facets and stages of female reproduction, being involved in processes such as follicle and oocyte maturation within the ovary, fertilization, embryo migration, implantation, and throughout the entire duration of pregnancy and birth(32). The impact of gut microbiota imbalances on such a huge list of conditions, that also involves infertility, polycystic ovary syndrome (PCOS), and endometriosis, has been extensively studied. In a recent investigation(33), researchers compared the gut-vaginal microbiota axis in fertile women to that of women diagnosed with RIF. Findings revealed that the infertile group exhibited reduced gut α-diversity, indicating the presence of low-grade inflammatory disorders. Further analysis unveiled a species composition shifts related to a weakened mucosal protection mechanism. When the integrity of the mucus barrier is compromised, gut bacteria and other microbe-associated molecular patterns trigger an immune response that can lead to both localized and systemic inflammation(34). In this intricate interplay, sex hormones serve as crucial mediators, facilitating communication between microorganisms and their host. Furthermore, the gut microbiome is recognized for its ability to modulate hormone levels, particularly influencing estrogen levels in females. Intestinal bacteria actively participate in estrogen metabolism through the secretion of β-glucuronidase (gmGUS), an enzyme that converts conjugated estrogen into its deconjugated form in the gastrointestinal tract. This conversion enables estrogen to bind to its receptors again, thereby initiating subsequent signaling cascades and estrogen-related physiological effects(35). The ensemble of gut microbiota genes responsible for estrogen metabolism is collectively referred to as the «estrobolome». In this context, reduced gmGUS activity due to gut microbial dysbiosis can lead to a diminished deconjugation of estrogen, resulting in lower circulating levels. The alteration in this enzyme activity also contribute to health issues such as obesity, cardiovascular pathologies and other diseases such as endometriosis(35).Thus, eubiosis maintenance is a key factor for hormone signaling.
Gut microbiome may also impact the levels of sex-steroid hormones in females indirectly, for instance, throughout the production of SCFAs. SCFAs stand as the primary by-products resulting from the anaerobic fermentation of dietary fibers by intestinal microbiome(36). This scenario shows a plausible mechanism through which dietary components and metabolites derived from the microbiota might contribute to the regulation of estrogen and progesterone levels in females. However, the precise molecular mechanisms underlying these interactions remain to be fully elucidated.
Inflammation exerts its influence on key events such as ovulation, menstruation, implantation, placentation, and pregnancy. Consequently, any disruption in the scale or duration of inflammatory events becomes a significant contributor to the pathophysiology of infertility(37), through mechanisms discussed in previous sections. However, the precise mechanism through which chronic low-grade inflammation hampers reproduction remains an area that requires further elucidation. On the contrary, it has been clearly depicted that chronic inflammation has the potential to disrupt the process of folliculogenesis by triggering oxidative stress(38). Moreover, chronic low-grade inflammation can also compromise ER. Notably, inflammatory conditions such as endometriosis, adenomyosis, and chronic endometritis rank among the leading causes of recurrent pregnancy loss. In patients with endometriosis, increased levels of inflammatory cytokines are found in the peritoneal fluid. This overexpression leads to heightened local estrogen production, ultimately disrupting ER(39).
Furthermore, oral probiotics can modulate the composition of the intestinal microbiota, improve intestinal integrity, and have an impact on the maintenance and recovery of the normal reproductive microbiota(27).
Future research efforts should prioritize uncovering the precise molecular mechanisms that underlie the connections between gut microbiota and reproductive diseases. Gaining a comprehensive understanding of this mutually influential relationship holds the potential to pave the way for the creation of innovative and impactful approaches for the prevention, early diagnosis, and treatment of female reproductive disorders.
Discussion
In recent years, the study of human endometrial microbiome has become a growing field of knowledge. IF can be due to various factors, such as the maternal immune system, the embryo genetics, anatomical factors, thrombotic factors or the reproductive microbiome, among others(33). A balance between immune response, resistance and immunogenic tolerance is important for embryo implantation and establishing a viable pregnancy. However, the extent of interactions between the microbiome and these immune responses remains unknown(29).
Alteration of the endometrial microbiome can affect the implantation process through different vias. On the one hand, the integrity of the endometrial mucosal barrier can be weakened. This weakness allows pathogens to colonize and an immune response to occur with an imbalance in the production of cytokines in favor of pro-inflammatory types. Furthermore, an aberration in the maturation of uNK cells and the alteration of macrophages balance may lead to an incomplete remodeling of the maternal spiral arteries(4,7). These events could alter ER, impair implantation process and the onset of a successful pregnancy(40). In addition, reproductive microbiome and its influence on immune response may play a fundamental role in highly prevalent diseases such as endometriosis(6).
The known interaction between other mucosal locations and endometrium includes translocations of metabolites, immune signaling and/or inflammation without knowing what the real impact is on endometrial functionality and eproductive pathologies(3,7,8). In this sense, and in order to delve deeper into the search for biomarkers at the reproductive microbiome level that are related to reproductive pathologies and clinical outcomes, it is necessary to reach a consensus on what is considered a normal or healthy microbiome in a multiethnic, global world. It is necessary to standardize different aspects such as sampling contamination, the molecular technique used, the data analysis for characterization, and the classification of "control" individuals based on their characteristics and medical clinical history(7).
Another important aspect to improve is the design of appropriate therapeutic strategies to treat the alteration of local microbiomes. For instance, vaginal microbiota transplantation has been shown to reduce the recurrence of bacterial vaginosis, opening a door to the possible success of uterine microbiota transplantation. However, it is still essential to consider the different aspects, ethical and technical ones, that will allow the clinical application of microbiome transplantation.
Furthermore, the use of probiotics, alone or in combination with antimicrobials, is a promising strategy. However, it is essential to determine which strains have the most therapeutic potential in each case. Different strains of Lactobacillus and their properties have been tested regarding their ability to restore bacterial balance at a reproductive level. However, it is necessary that the probiotic strain has the ability to colonize the uterus and that it be isolated from healthy fertile women for the achievement of future accurate clinical trials(21). The use of these probiotics can avoid the drawbacks of antibiotic use, such as antibiotic resistance or the elimination of microbiota from other locations(7).
Furthermore, many other questions remain to be resolved, such as the role of specific microorganisms on clinical reproductive outcomes and the interaction of the microbiome with endocrine regulation(7). In addition, establishing what is considered a eubiotic microenvironment taking into account the analysis technique used, among other aspects, will allow a better understanding of the physiological profile of the endometrium and will reduce the overconsideration of the dysbiotic state and the treatment failure rates(12).
In short, it is essential to carry out a joint study of the reproductive microbiome and the immunological profile for the management of infertility in patients with an indication for endometrial evaluation. Personalized treatment of the microbiota of the reproductive tract with more and more specific treatments could improve the clinical success. Current advances in research even allow us to study the presence of antibiotic resistance genes to facilitate the management of recurrent infections or the treatment of microorganisms with high levels of resistance to wide ranges of antibiotic groups(10).
Well-designed clinical studies on the importance of certain microorganisms, including those less studied such as fungi and viruses, in reproductive results and on the effectiveness of different therapeutic strategies will allow the resolution of infertility in numerous clinical cases and the improve in reproductive health.



