

ISSN 2954-467X

Hannah E Pierson PhD
1 Synergyne Imaging Technology, Inc., Canmore, Alberta, Canada.
2 Obstetrics and Gynecology, College of Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
NOTE: The numbers following the affiliation markers are the author's ORCID iD.
Correspondence: Roger A. Pierson. MS, PhD, FEAS, FCAHS. mail: roger.pierson@usask.ca.
Article History: Received June 14, 2024. Revised June 20, 2024. Accepted July 13, 2024. Available online August 12, 2024.
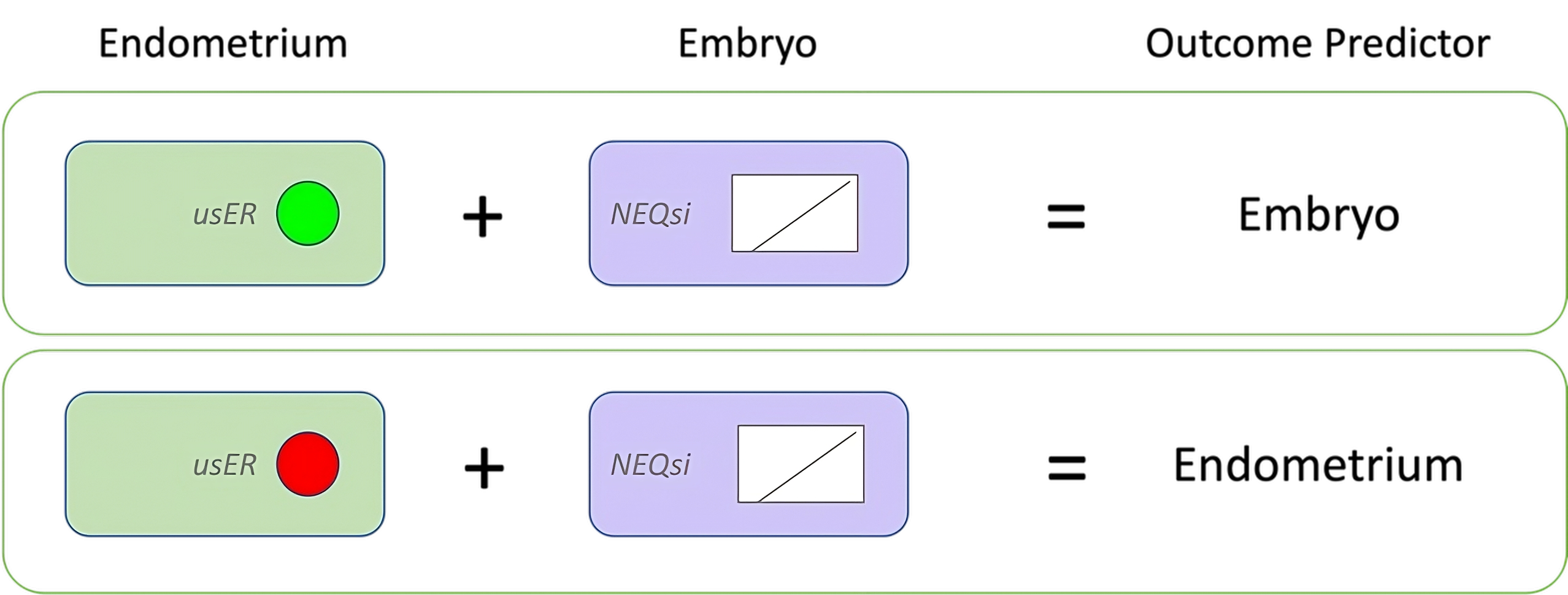
Figure 1. Linking Embryo quality and ER. The green circle (upper left) represents receptive endometria and red circle (lower left) represents poor-receptivity endometria, as assessed by usER. The range and distribution of NEQsi scores were comparable between the two groups. The outcome predictor for each subanalysis is shown on the right.
[1] Yildiz S, Yakin K, Ata B, Oktem O. There is a cycle to cycle variation in ovarian response and pre-hCG serum progesterone level: an analysis of 244 consecutive IVF cycles. Sci Rep. 2020;10(1):15793.
[2] Haham LM, Shani AK, Roumia A, Kuznyetsove I, Madjunkov M, Librach C. Intra-patient inter-cycle variation analysis - does the outcome of a prior IVF cycle predict the outcome of subsequent cycle(s)? Fertil and Steril. 2021;116(3):E234.
[3] Baerwald AR, Pierson RA. Endometrial development in association with ovarian follicular waves during the menstrual cycle. Ultrasound Obstet Gynecol. 2004;24(4):453-60.
[4] Liu KE, Hartman M, Hartman A, Luo ZC, Mahutte N. The impact of a thin endometrial lining on fresh and frozen-thaw IVF outcomes: an analysis of over 40 000 embryo transfers. Hum Reprod. 2018;33(10):1883-8.
[5] Mahutte N, Hartman M, Meng L, Lanes A, Luo ZC, Liu KE. Optimal endometrial thickness in fresh and frozen-thaw in vitro fertilization cycles: an analysis of live birth rates from 96,000 autologous embryo transfers. Fertil Steril. 2022;117(4):792-800.
[6] Mathyk B, Schwartz A, DeCherney A, Ata B. A critical appraisal of studies on endometrial thickness and embryo transfer outcome. Reprod Biomed Online. 2023;47(4):103259.
[7] Applebaum M. The Uterine Biophysical Profile. Ultrasound in Obstetrics and Gynecology. 1995;5(1):67-8.
[8] Farshchian N, Fakheri T, Bahrami Kamangar P, Lorestani H, Azadbakht J. Pregnancy rate in intrauterine insemination, is uterine biophysical profile of predictive value? A prospective study. J Ultrasound. 2022;25(4):949-55.
[9] Asch RH, Alkon T, Zamora Ramirez ML, Suarez J, Luagas N. Ultrasonographic endometrial classification in In Vitro Fertiliztion: a new approach. JIVFww. 2023;1(1-3).
[10] EH N, PC H. Ultrasound Assessment of Endometrial Recptivity in in vitro Fertilization Treatment. Donald School Journal of Ultrasound in Obstetrics and Gynegology. 2010;4(2):179-88.
[11] Pierson RA. Imaging the endometrium: are there predictors of uterine receptivity? J Obstet Gynaecol Can. 2003;25(5):360-8.
[12] Killick SR. Ultrasound and the receptivity of the endometrium. Reprod Biomed Online. 2007;15(1):63-7.
[13] Steer CV, Campbell S, Tan SL, Crayford T, Mills C, Mason BA, et al. The use of transvaginal color flow imaging after in vitro fertilization to identify optimum uterine conditions before embryo transfer. Fertil Steril. 1992;57(2):372-6.
[14] Bahrami F, Eftekhar M, Zanbagh L. Uterine artery Doppler and endometrial blood flow in frozen embryo transfer: A cohort study. Int J Reprod Biomed. 2023;21(3):205-12.
[15] Fanchin R, Righini C, Olivennes F, Taylor S, de Ziegler D, Frydman R. Uterine contractions at the time of embryo transfer alter pregnancy rates after in-vitro fertilization. Hum Reprod. 1998;13(7):1968-74.
[16] Ng EH, Li RH, Chen L, Lan VT, Tuong HM, Quan S. A randomized double blind comparison of atosiban in patients undergoing IVF treatment. Hum Reprod. 2014;29(12):2687-94.
[17] Franchin R, Ayoubi JM, Righini C, Olivennes F, Schonauer LM, Frydman R. Uterine contractility decreases at the time of blastocyst trasnfers. Human Reproduction. 2001;16(6):1115-9.
[18] Maged AM, Ramzy AM, Ghar MA, El Shenoufy H, Gad Allah SH, Wahba AH, et al. 3D ultrasound assessment of endometrial junctional zone anatomy as a predictor of the outcome of ICSI cycles. Eur J Obstet Gynecol Reprod Biol. 2017;212:160-5.
[19] Raga F, Bonilla-Musoles, Casan EM, Klein O, Bonilla F. Assessment of the endometrial volume by three-dimensional ultrasound prior to embryo transfer. Human Reproduction. 1999;14(11):2851-4.
[20] Silva Martins R, Helio Oliani A, Vaz Oliani D, Martinez de Oliveira J. The predictive value of serial serum estradiol and serial endometrial volume on endometrial receptivity on assisted reproductive technology cycles. BMC Pregnancy Childbirth. 2021;21(1):184.
[21] Zollner U, Specketer MT, Dietl J, Zollner KP. 3D-Endometrial volume and outcome of cryopreserved embryo replacement cycles. Arch Gynecol Obstet. 2012;286(2):517-23.
[22] Martins RS, Oliani AH, Oliani DV, de Oliveira JM. Continuous endometrial volumetric analysis for endometrial receptivity assessment on assisted reproductive technology cycles. BMC Pregnancy Childbirth. 2020;20(1):663.
[23] Elsokkary M, Eldin AB, Abdelhafez M, Rateb A, Samy M, Eldorf A, et al. The reproducibility of the novel utilization of five-dimensional ultrasound and power Doppler in the prediction of endometrial receptivity in intracytoplasmic sperm-injected women: a pilot prospective clinical study. Arch Gynecol Obstet. 2019;299(2):551-8.
[24] Maged AM, Kamel AM, Abu-Hamila F, Elkomy RO, Ohida OA, Hassan SM, et al. The measurement of endometrial volume and sub-endometrial vascularity to replace the traditional endometrial thickness as predictors of in-vitro fertilization success. Gynecol Endocrinol. 2019;35(11):949-54.
[25] Mayer RB, Ebner T, Weiss C, Allerstorfer C, Altmann R, Oppelt P, et al. The Role of Endometrial Volume and Endometrial and Subendometrial Vascularization Parameters in a Frozen Embryo Transfer Cycle. Reprod Sci. 2019;26(7):1013-8.
[26] Schlid RL, Indefrei D, Eschweiler S, Van der Ven H, Fimmers R, Hansmann M. Three-dimensional endometrial volume calculation and pregnancy rate in an in-vitro fertiliztion programme. Human Reproduction. 1999;14(5):1255-8.
[27] Nayyef SA, Abdullah TH, Al Obaidi MT. Accuracy of endometrial length measurement in predicting IVF/ICSI outcome. J Med Life. 2022;15(9):1176-80.
[28] Ahmadi F, Maghari A, Pahlavan F. Predictive Value of Endometrial Length Measurement by Transvaginal Ultrasound and IVF/ICSI Outcomes. Int J Fertil Steril. 2020;14(3):209-12.
[29] Haas J, Smith R, Zilberberg E, Nayot D, Meriano J, Barzilay E, et al. Endometrial compaction (decreased thickness) in response to progesterone results in optimal pregnancy outcome in frozen-thawed embryo transfers. Fertil Steril. 2019;112(3):503-9 e1.
[30] Zilberberg E, Smith R, Nayot D, Haas J, Meriano J, Barzilay E, et al. Endometrial compaction before frozen euploid embryo transfer improves ongoing pregnancy rates. Fertil Steril. 2020;113(5):990-5.
[31] Shah JS, Vaughan DA, Dodge LE, Leung A, Korkidakis A, Sakkas D, et al. Endometrial compaction does not predict live birth in single euploid frozen embryo transfers: a prospective study. Hum Reprod. 2022;37(5):980-7.
[32] Youngster M, Mor M, Kedem A, Gat I, Yerushalmi G, Gidoni Y, et al. Endometrial compaction is associated with increased clinical and ongoing pregnancy rates in unstimulated natural cycle frozen embryo transfers: a prospective cohort study. J Assist Reprod Genet. 2022;39(8):1909-16.
[33] Yaprak E, Sukur YE, Ozmen B, Sonmezer M, Berker B, Atabekoglu C, et al. Endometrial compaction is associated with the increased live birth rate in artificial frozen-thawed embryo transfer cycles. Hum Fertil (Camb). 2021:1-7.
[34] Ju W, Wei C, Lu X, Zhao S, Song J, Wang H, et al. Endometrial compaction is associated with the outcome of artificial frozen-thawed embryo transfer cycles: a retrospective cohort study. J Assist Reprod Genet. 2023;40(7):1649-60.
[35] Olgan S, Dirican EK, Sakinci M, Caglar M, Ozsipahi AC, Gul SM, et al. Endometrial compaction does not predict the reproductive outcome after vitrified-warmed embryo transfer: a prospective cohort study. Reprod Biomed Online. 2022;45(1):81-7.
[36] Erdogan K, Sanlier NT, Utlu Ozen E, Dilbaz S, Kahyaoglu I, Ustun Y. Investigating the impact of endometrial compaction on clinical pregnancy rate in artifical frozen-thawed embryo transfer cycles. Marmara Med J. 2023;36(1):34-8.
[37] Bu Z, Yang X, Song L, Kang B, Sun Y. The impact of endometrial thickness change after progesterone administration on pregnancy outcome in patients transferred with single frozen-thawed blastocyst. Reprod Biol Endocrinol. 2019;17(1):99.
[38] Huang J, Lin J, Cai R, Lu X, Song N, Gao H, et al. Significance of endometrial thickness change after human chorionic gonadotrophin triggering in modified natural cycles for frozen-thawed embryo transfer. Ann Transl Med. 2020;8(23):1590.
[39] Huang J, Lin J, Gao H, Zhu J, Lu X, Song N, et al. Value of endometrial thickness change after human chorionic gonadotrophin administration in predicting pregnancy outcome following fresh transfer in vitro fertilization cycles. Arch Gynecol Obstet. 2021;303(2):565-72.
[40] Pierson RM, M.; Kuzcynskiw, W.; Klein, B.; Arce, J.; . Endometrial quality at the end of controlled ovarian stimulation predicts ongoing pregnancy rate after transfer of a single expanded or hatching/hatched blastocyst on day 5 in a fresh cycle. Fertil Steril. 2012;98(4):S225.
[41] Pierson RA. Unpublished Data. 2014.
[42] Pierson HE, Cadesky K, Meriano J, Invik J, Laskin C, Pierson RA. Ultrasound Based Endometrial Receptivity Scoring Improves In Vitro Fertilization Pregnancy Rates. Journal of Fertilization: IVF Worldwide. 2021;9(6):248.
[43] Cadesky K PH, Laskin CA, Meriano J, Invik J, Pierson RA. Ultrasound Image-Based Scoring System Improves IVF Pregnancy Rates. Proceedings of the Annual Conference of the Canadian Fertility and Andrology Society. 2019;19-21:p121.
[44] Samara N, Casper RF, Bassil R, Shere M, Barzilay E, Orvieto R, et al. Sub-endometrial contractility or computer-enhanced 3-D modeling scoring of the endometrium before embryo transfer: are they better than measuring endometrial thickness? J Assist Reprod Genet. 2019;36(1):139-43.
[45] Pierson HE, Cadesky K, Meriano J, Invik J, Laskin CA, Pierson RA. Ultrasound based endometrial receptivity scoring accurately identifies IVF cycles with low probability of pregnancy. Fertil Steril. 2021;116(3):E-312.
[46] Cadesky K, Pierson HE, Invik J, Meriano J, laskin CA, Pierson RA. Building better endometria for IVF: usER testing and clinical trends in endometrial quality over time. Proceedings of the Annual Conference of the Canadian Fertility and Andrology Society. 2023.
[47] Pierson HE, Invik J, Meriano J, Pierson RA. A novel system for rapid conversion of Gardner embryo grades to linear scale numeric variables. Reprod Biomed Online. 2023;46(5):808-18.
[48] Liu Y, Zhou Q, Peng B, Jiang J, Fang L, Weng W, et al. Automatic Measurement of Endometrial Thickness From Transvaginal Ultrasound Images. Front Bioeng Biotechnol. 2022;10:853845.
[49] Wang X, Bao N, Xin X, Tan J, Li H, Zhou S, et al. Automatic evaluation of endometrial receptivity in three-dimensional transvaginal ultrasound images based on 3D U-Net segmentation. Quant Imaging Med Surg. 2022;12(8):4095-108.
[50] Park H, Lee HJ, Kim HG, Ro YM, Shin D, Lee SR, et al. Endometrium segmentation on transvaginal ultrasound image using key-point discriminator. Med Phys. 2019;46(9):3974-84.
[51] Liang X, He J, He L, Lin Y, Li Y, Cai K, et al. An ultrasound-based deep learning radiomic model combined with clinical data to predict clinical pregnancy after frozen embryo transfer: a pilot cohort study. Reprod Biomed Online. 2023;47(2):103204.